Sodium Tanshinone IIA Sulfonate Inhibits Vascular Endothelial Cell Pyroptosis via the AMPK Signaling Pathway in Atherosclerosis
- PMID: 36408328
- PMCID: PMC9673812
- DOI: 10.2147/JIR.S386470
Sodium Tanshinone IIA Sulfonate Inhibits Vascular Endothelial Cell Pyroptosis via the AMPK Signaling Pathway in Atherosclerosis
Abstract
Introduction: Atherosclerosis (AS) is the underlying cause of cardiovascular events. Endothelial cell mitochondrial damage and pyroptosis are important factors contributing to AS. Changes in internal mitochondrial conformation and increase in reactive oxygen species (ROS) lead to the disruption of mitochondrial energy metabolism, activation of the NLRP3 inflammasome and pyroptosis, which in turn affect atherogenesis by impairing endothelial function. AMPK is a core player in the regulation of cellular metabolism, not only by regulating mitochondrial homeostasis but also by regulating cellular inflammatory responses. Sodium tanshinone IIA sulfonate (STS), a water-soluble derivative of tanshinone IIA, has significant antioxidant and anti-inflammatory effects, and roles in cardiovascular protection.
Purpose: In this study, we investigated whether STS plays a protective role in AS by regulating endothelial cell mitochondrial function and pyroptosis through an AMPK-dependent mitochondrial pathway.
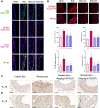
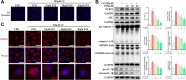
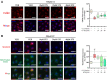
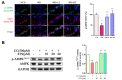
Methods and results: Male ApoE-/- mice and HUVECs were used for the experiments. We found that STS treatment largely abrogated the upregulation of key proteins in aortic vessel wall plaques and typical pyroptosis signaling in ApoE-/- mice fed a western diet, consequently enhancing pAMPK expression, plaque stabilization, and anti-inflammatory responses. Consistently, STS pretreatment inhibited cholesterol crystallization (CC) -induced cell pyroptosis and activated pAMPK expression. In vitro, using HUVECs, we further found that STS treatment ameliorated mitochondrial ROS caused by CC, as evidenced by the finding that STS inhibited mitochondrial damage caused by CC. The improvement of endothelial cell mitochondrial function by STS is blocked by dorsomorphin (AMPK inhibitor). Consistently, the blockade of endothelial cell pyroptosis by STS is disrupted by dorsomorphin.
Conclusion: Our results suggest that STS enhances maintenance of mitochondrial homeostasis and inhibits mitochondrial ROS overproduction via AMPK, thereby improving endothelial cell pyroptosis during AS.
Keywords: AMPK; atherosclerosis; mitochondria; pyroptosis; tanshinone IIA.
© 2022 Zhu et al.
Conflict of interest statement
The authors report no conflicts of interest in relation to this work and declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Tanshinone IIA: a Chinese herbal ingredient for the treatment of atherosclerosis.Front Pharmacol. 2023 Dec 1;14:1321880. doi: 10.3389/fphar.2023.1321880. eCollection 2023. Front Pharmacol. 2023. PMID: 38108067 Free PMC article. Review.
-
Prevention of endotoxin-induced cardiomyopathy using sodium tanshinone IIA sulfonate: Involvement of augmented autophagy and NLRP3 inflammasome suppression.Eur J Pharmacol. 2021 Oct 15;909:174438. doi: 10.1016/j.ejphar.2021.174438. Epub 2021 Aug 23. Eur J Pharmacol. 2021. PMID: 34437885
-
Tanshinone IIA Sodium sulfonate regulates antioxidant system, inflammation, and endothelial dysfunction in atherosclerosis by downregulation of CLIC1.Eur J Pharmacol. 2017 Nov 15;815:427-436. doi: 10.1016/j.ejphar.2017.09.047. Epub 2017 Sep 29. Eur J Pharmacol. 2017. PMID: 28970012
-
Sodium tanshinone IIA sulfonate protects vascular relaxation in ApoE-knockout mice by inhibiting the SYK-NLRP3 inflammasome-MMP2/9 pathway.BMC Cardiovasc Disord. 2024 Jul 12;24(1):354. doi: 10.1186/s12872-024-03990-0. BMC Cardiovasc Disord. 2024. PMID: 38992615 Free PMC article.
-
Sodium tanshinone IIA sulfonate: A review of pharmacological activity and pharmacokinetics.Biomed Pharmacother. 2019 Oct;118:109362. doi: 10.1016/j.biopha.2019.109362. Epub 2019 Aug 24. Biomed Pharmacother. 2019. PMID: 31545252 Review.
Cited by
-
Targeting mitochondria by lipid-selenium conjugate drug results in malate/fumarate exhaustion and induces mitophagy-mediated necroptosis suppression.Int J Biol Sci. 2024 Oct 28;20(14):5793-5811. doi: 10.7150/ijbs.102424. eCollection 2024. Int J Biol Sci. 2024. PMID: 39494338 Free PMC article.
-
Proteins and DNA Sequences Interacting with Tanshinones and Tanshinone Derivatives.Int J Mol Sci. 2025 Jan 20;26(2):848. doi: 10.3390/ijms26020848. Int J Mol Sci. 2025. PMID: 39859562 Free PMC article. Review.
-
Harnessing the therapeutic value of Tanshinone IIA: a breakthrough therapy in cardiovascular diseases.Front Pharmacol. 2025 Jul 4;16:1620152. doi: 10.3389/fphar.2025.1620152. eCollection 2025. Front Pharmacol. 2025. PMID: 40689206 Free PMC article. Review.
-
Tanshinone IIA: a Chinese herbal ingredient for the treatment of atherosclerosis.Front Pharmacol. 2023 Dec 1;14:1321880. doi: 10.3389/fphar.2023.1321880. eCollection 2023. Front Pharmacol. 2023. PMID: 38108067 Free PMC article. Review.
-
ROS induced pyroptosis in inflammatory disease and cancer.Front Immunol. 2024 Jul 1;15:1378990. doi: 10.3389/fimmu.2024.1378990. eCollection 2024. Front Immunol. 2024. PMID: 39011036 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

