Profile of Tirbanibulin for the Treatment of Actinic Keratosis
- PMID: 36408375
- PMCID: PMC9586524
Profile of Tirbanibulin for the Treatment of Actinic Keratosis
Abstract
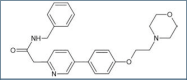
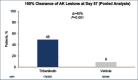
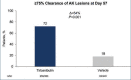
Actinic keratosis (AK) is a chronic disease resulting from deleterious effects of long-term, cumulative, epidermal exposure to ultraviolet (UV) light. UV-induced mutations in p53, ras, and p16 genes lead to the emergence of abnormal epidermal actinic keratosis (AKs) cells, which proliferate while avoiding apoptosis and may lead to invasive squamous cell carcinoma. There are both lesion-targeted and field-directed topical treatments. This review is of new and emerging information on tirbanibulin and tirbanibulin 1% ointment, which is approved for topical field treatment of actinic keratosis on the face and scalp. Potent antiproliferative and proapoptotic activities result from tirbanibulin's inhibition tubulin polymerization and disruption of microtubule formation and Src kinase signaling. Tirbanibulin 1% ointment is an effective treatment of facial and scalp AK after five consecutive once-daily applications, as measured by complete and partial clearance and percent reduction in the number of lesions. Localized skin reactions are usually mild to moderate, resolving within a month. The short and well-tolerated course of therapy results in very high patient adherence to the treatment regimen.
Keywords: Tirbanibulin ointment; actinic keratosis; field therapy.
Copyright © 2022. Matrix Medical Communications. All rights reserved.
Conflict of interest statement
FINANCIAL DISCLOSURES: Dr. B. Berman is an advisory board member, investigator, consultant, and/or speaker for Biofrontera, SUN Pharmaceuticals, Inc., LEO Pharma, and Almirall LLC. Dr. Grada is a former Almirall LLC employee. Ms. D. Berman has no conflicts of interest relevant to the content of this article.
Figures

Similar articles
-
Tirbanibulin 1% Ointment: The Mechanism of Action of a Novel Topical Therapy for Actinic Keratosis.J Drugs Dermatol. 2025 Jul 1;24(7):19913s13-19913s18. doi: 10.36849/JDD.19913. J Drugs Dermatol. 2025. PMID: 40627585
-
Tirbanibulin Ointment 1% as a Novel Treatment for Actinic Keratosis: Phase 1 and 2 Results.J Drugs Dermatol. 2020 Nov 1;19(11):1093-1100. doi: 10.36849/JDD.2020.5576. J Drugs Dermatol. 2020. PMID: 33196758 Clinical Trial.
-
Phase 3 Trials of Tirbanibulin Ointment for Actinic Keratosis.N Engl J Med. 2021 Feb 11;384(6):512-520. doi: 10.1056/NEJMoa2024040. N Engl J Med. 2021. PMID: 33567191 Clinical Trial.
-
Tirbanibulin for the Treatment of Actinic Keratosis: A Review.Skin Therapy Lett. 2022 Jul;27(4):4-7. Skin Therapy Lett. 2022. PMID: 35857917 Review.
-
Clinical and experimental aspects of tirbanibulin treatments.Arch Dermatol Res. 2025 Mar 19;317(1):603. doi: 10.1007/s00403-025-04119-9. Arch Dermatol Res. 2025. PMID: 40105986 Review.
Cited by
-
Topical Treatments for Basal Cell Carcinoma and Actinic Keratosis in the United States.Cancers (Basel). 2023 Aug 2;15(15):3927. doi: 10.3390/cancers15153927. Cancers (Basel). 2023. PMID: 37568743 Free PMC article. Review.
-
Systematic review of randomized controlled trials of topicals for actinic keratosis field therapy.Arch Dermatol Res. 2024 Mar 18;316(4):108. doi: 10.1007/s00403-024-02839-y. Arch Dermatol Res. 2024. PMID: 38498070
References
-
- Drake LA, Ceilley RI, Cornelison RL et al. Guidelines of care for actinic keratoses. J Am Acad Dermatol. 1995;32(1):95–98. - PubMed
-
- https://www.lewin.com/content/dam/Lewin/Resources/Site_Sections/Publicat... Society for Investigative Dermatology, American Academy of Dermatology Association. The burden of skin diseases, 2005. Accessed 2 Feb 2022. - PubMed
-
- Cockerell CJ, Wharton JR. New histopathological classification of actinic keratosis (incipient intraepidermal squamous cell carcinoma). J Drug Dermatol. 2005;4(4):462–467. - PubMed
-
- Berman B, Bienstock L, Kuritzky L et al. Actinic keratosis: sequelae and treatments: recommendations from a consensus panel. J Fam Pract. 2006;55(5):S1–S1. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
