Exploring the pathogenesis of age-related macular degeneration: A review of the interplay between retinal pigment epithelium dysfunction and the innate immune system
- PMID: 36408381
- PMCID: PMC9670140
- DOI: 10.3389/fnins.2022.1009599
Exploring the pathogenesis of age-related macular degeneration: A review of the interplay between retinal pigment epithelium dysfunction and the innate immune system
Abstract
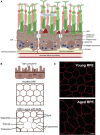
Age-related macular degeneration (AMD) is a leading cause of irreversible vision loss in the older population. Classical hallmarks of early and intermediate AMD are accumulation of drusen, a waste deposit formed under the retina, and pigmentary abnormalities in the retinal pigment epithelium (RPE). When the disease progresses into late AMD, vision is affected due to death of the RPE and the light-sensitive photoreceptors. The RPE is essential to the health of the retina as it forms the outer blood retinal barrier, which establishes ocular immune regulation, and provides support for the photoreceptors. Due to its unique anatomical position, the RPE can communicate with the retinal environment and the systemic immune environment. In AMD, RPE dysfunction and the accumulation of drusen drive the infiltration of retinal and systemic innate immune cells into the outer retina. While recruited endogenous or systemic mononuclear phagocytes (MPs) contribute to the removal of noxious debris, the accumulation of MPs can also result in chronic inflammation and contribute to AMD progression. In addition, direct communication and indirect molecular signaling between MPs and the RPE may promote RPE cell death, choroidal neovascularization and fibrotic scarring that occur in late AMD. In this review, we explore how the RPE and innate immune cells maintain retinal homeostasis, and detail how RPE dysfunction and aberrant immune cell recruitment contribute to AMD pathogenesis. Evidence from AMD patients will be discussed in conjunction with data from preclinical models, to shed light on future therapeutic targets for the treatment of AMD.
Keywords: age related macular degeneration (AMD); dendritic cell; inflammation; macrophage; microglia; mononuclear phagocyte (MP); para-inflammation; retinal pigment epithelium (RPE).
Copyright © 2022 Wong, Ma, Jobling, Brandli, Greferath, Fletcher and Vessey.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Ach T., Tolstik E., Messinger J. D., Zarubina A. V., Heintzmann R., Curcio C. A. (2015). Lipofuscin redistribution and loss accompanied by cytoskeletal stress in retinal pigment epithelium of eyes with age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 56 3242–3252. 10.1167/iovs.14-16274 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

