Upregulation of neuropeptide Y in cardiac sympathetic nerves induces stress (Takotsubo) cardiomyopathy
- PMID: 36408384
- PMCID: PMC9669346
- DOI: 10.3389/fnins.2022.1013712
Upregulation of neuropeptide Y in cardiac sympathetic nerves induces stress (Takotsubo) cardiomyopathy
Abstract
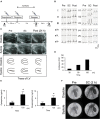
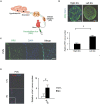
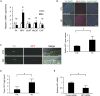
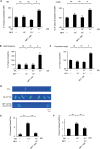
Substantial emotional or physical stress may lead to an imbalance in the brain, resulting in stress cardiomyopathy (SC) and transient left ventricular (LV) apical ballooning. Even though these conditions are severe, their precise underlying mechanisms remain unclear. Appropriate animal models are needed to elucidate the precise mechanisms. In this study, we established a new animal model of epilepsy-induced SC. The SC model showed an increased expression of the acute phase reaction protein, c-Fos, in the paraventricular hypothalamic nucleus (PVN), which is the sympathetic nerve center of the brain. Furthermore, we observed a significant upregulation of neuropeptide Y (NPY) expression in the left stellate ganglion (SG) and cardiac sympathetic nerves. NPY showed neither positive nor negative inotropic and chronotropic effects. On the contrary, NPY could interrupt β-adrenergic signaling in cardiomyocytes when exposure to NPY precedes exposure to noradrenaline. Moreover, its elimination in the left SG via siRNA treatment tended to reduce the incidence of SC. Thus, our results indicated that upstream sympathetic activation induced significant upregulation of NPY in the left SG and cardiac sympathetic nerves, resulting in cardiac dysfunctions like SC.
Keywords: Takotsubo cardiomyopathy; cardiac sympathetic nervous; neuropeptide Y; stellate ganglion; stress.
Copyright © 2022 Arai, Kanazawa, Kimura, Munakata, Yamakawa, Shinmura, Yuasa, Sano and Fukuda.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Anderson J. L., Adams C. D., Antman E. M., Bridges C. R., Califf R. M., Casey D. E., Jr., et al. (2007). ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: A report of the American college of cardiology/American heart association task force on practice guidelines (Writing committee to revise the 2002 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction) developed in collaboration with the American college of emergency physicians, the society for cardiovascular angiography and interventions, and the society of thoracic surgeons endorsed by the American association of cardiovascular and pulmonary rehabilitation and the society for academic emergency medicine. J. Am. Coll. Cardiol. 50 e1–e157. 10.1016/j.jacc.2007.02.013 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

