Autism spectrum disorders pathogenesis: Toward a comprehensive model based on neuroanatomic and neurodevelopment considerations
- PMID: 36408388
- PMCID: PMC9671112
- DOI: 10.3389/fnins.2022.988735
Autism spectrum disorders pathogenesis: Toward a comprehensive model based on neuroanatomic and neurodevelopment considerations
Abstract
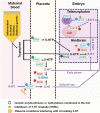
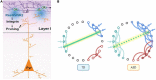
Autism spectrum disorder (ASD) involves alterations in neural connectivity affecting cortical network organization and excitation to inhibition ratio. It is characterized by an early increase in brain volume mediated by abnormal cortical overgrowth patterns and by increases in size, spine density, and neuron population in the amygdala and surrounding nuclei. Neuronal expansion is followed by a rapid decline from adolescence to middle age. Since no known neurobiological mechanism in human postnatal life is capable of generating large excesses of frontocortical neurons, this likely occurs due to a dysregulation of layer formation and layer-specific neuronal migration during key early stages of prenatal cerebral cortex development. This leads to the dysregulation of post-natal synaptic pruning and results in a huge variety of forms and degrees of signal-over-noise discrimination losses, accounting for ASD clinical heterogeneities, including autonomic nervous system abnormalities and comorbidities. We postulate that sudden changes in environmental conditions linked to serotonin/kynurenine supply to the developing fetus, throughout the critical GW7 - GW20 (Gestational Week) developmental window, are likely to promote ASD pathogenesis during fetal brain development. This appears to be driven by discrete alterations in differentiation and patterning mechanisms arising from in utero RNA editing, favoring vulnerability outcomes over plasticity outcomes. This paper attempts to provide a comprehensive model of the pathogenesis and progression of ASD neurodevelopmental disorders.
Keywords: RNA editing; autism spectrum disorder (ASD); autonomic nervous system (ANS); kynurenine metabolites; maternal inflammation; neurodevelopment; serotonin; synaptic pruning.
Copyright © 2022 Beopoulos, Géa, Fasano and Iris.
Conflict of interest statement
AB, MG, and FI were employed by the company Bio-Modeling Systems. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






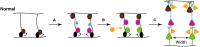
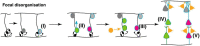

References
LinkOut - more resources
Full Text Sources

