Fmrp regulates neuronal balance in embryonic motor circuit formation
- PMID: 36408418
- PMCID: PMC9669763
- DOI: 10.3389/fnins.2022.962901
Fmrp regulates neuronal balance in embryonic motor circuit formation
Abstract
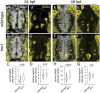
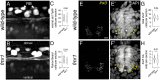
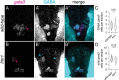
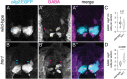
Motor behavior requires the balanced production and integration of a variety of neural cell types. Motor neurons are positioned in discrete locations in the spinal cord, targeting specific muscles to drive locomotive contractions. Specialized spinal interneurons modulate and synchronize motor neuron activity to achieve coordinated motor output. Changes in the ratios and connectivity of spinal interneurons could drastically alter motor output by tipping the balance of inhibition and excitation onto target motor neurons. Importantly, individuals with Fragile X syndrome (FXS) and associated autism spectrum disorders often have significant motor challenges, including repetitive behaviors and epilepsy. FXS stems from the transcriptional silencing of the gene Fragile X Messenger Ribonucleoprotein 1 (FMR1), which encodes an RNA binding protein that is implicated in a multitude of crucial neurodevelopmental processes, including cell specification. Our work shows that Fmrp regulates the formation of specific interneurons and motor neurons that comprise early embryonic motor circuits. We find that zebrafish fmr1 mutants generate surplus ventral lateral descending (VeLD) interneurons, an early-born cell derived from the motor neuron progenitor domain (pMN). As VeLD interneurons are hypothesized to act as central pattern generators driving the earliest spontaneous movements, this imbalance could influence the formation and long-term function of motor circuits driving locomotion. fmr1 embryos also show reduced expression of proteins associated with inhibitory synapses, including the presynaptic transporter vGAT and the postsynaptic scaffold Gephyrin. Taken together, we show changes in embryonic motor circuit formation in fmr1 mutants that could underlie persistent hyperexcitability.
Keywords: Fragile X syndrome; GABAergic interneurons; cell fate specification; motor circuits; motor neuron development; synapse development.
Copyright © 2022 Barker, Miles and Doll.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Fmrp regulates oligodendrocyte lineage cell specification and differentiation.Glia. 2021 Oct;69(10):2349-2361. doi: 10.1002/glia.24041. Epub 2021 Jun 10. Glia. 2021. PMID: 34110049 Free PMC article.
-
Neuron-Specific FMRP Roles in Experience-Dependent Remodeling of Olfactory Brain Innervation during an Early-Life Critical Period.J Neurosci. 2021 Feb 10;41(6):1218-1241. doi: 10.1523/JNEUROSCI.2167-20.2020. Epub 2021 Jan 5. J Neurosci. 2021. PMID: 33402421 Free PMC article.
-
Chloride imbalance in Fragile X syndrome.Front Neurosci. 2022 Oct 12;16:1008393. doi: 10.3389/fnins.2022.1008393. eCollection 2022. Front Neurosci. 2022. PMID: 36312023 Free PMC article.
-
BDNF in fragile X syndrome.Neuropharmacology. 2014 Jan;76 Pt C:729-36. doi: 10.1016/j.neuropharm.2013.05.018. Epub 2013 May 29. Neuropharmacology. 2014. PMID: 23727436 Review.
-
Fragile X Syndrome as an interneuronopathy: a lesson for future studies and treatments.Front Neurosci. 2023 Apr 28;17:1171895. doi: 10.3389/fnins.2023.1171895. eCollection 2023. Front Neurosci. 2023. PMID: 37188005 Free PMC article. Review.
Cited by
-
Increased body weight in mice with fragile X messenger ribonucleoprotein 1 (Fmr1) gene mutation is associated with hypothalamic dysfunction.Sci Rep. 2023 Aug 4;13(1):12666. doi: 10.1038/s41598-023-39643-z. Sci Rep. 2023. PMID: 37542065 Free PMC article.
-
Creation of a novel zebrafish model with low DHA status to study the role of maternal nutrition during neurodevelopment.J Lipid Res. 2025 Jan;66(1):100716. doi: 10.1016/j.jlr.2024.100716. Epub 2024 Nov 27. J Lipid Res. 2025. PMID: 39608569 Free PMC article.
-
Electrical Synapses Mediate Embryonic Hyperactivity in a Zebrafish Model of Fragile X Syndrome.J Neurosci. 2024 Jul 31;44(31):e2275232024. doi: 10.1523/JNEUROSCI.2275-23.2024. J Neurosci. 2024. PMID: 38969506 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

