Absence of antibody responses to SARS-CoV-2 N protein in COVID-19 vaccine breakthrough cases
- PMID: 36408542
- PMCID: PMC9679329
- DOI: 10.1177/15353702221134097
Absence of antibody responses to SARS-CoV-2 N protein in COVID-19 vaccine breakthrough cases
Abstract
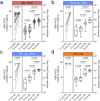
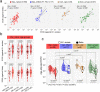
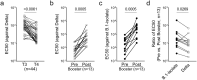
Understanding the risk factors for breakthrough coronavirus disease 2019 (COVID-19) (BC19) is critical to inform policy. Herein, we assessed Delta (Lineage B.1.617.2) variant-specific effectiveness of the BNT162b2 (Pfizer) vaccine and characterized Delta-driven BC19 cases (fully vaccinated individuals who get infected) with known-time-since-vaccination. In this longitudinal prospective study (January 21-October 30, 2021), 90 naïve and 15 convalescent individuals were enrolled at the initiation of vaccination. Samples from 27 unvaccinated individuals with previous laboratory-confirmed COVID-19 diagnosis were collected at a single time point. Longitudinal serology profile (antibodies against severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2] S and N proteins) and live-virus-based neutralization capacities were assessed while controlling for age. Sex, age, history of reactions to the COVID-19 vaccine, and viral neutralization capacities were identified as significant risk factors for breakthrough COVID-19. At 8 months postvaccination, male sex, individuals ⩾65 years of age, and individuals who experienced noticeable side effects with the COVID-19 vaccine were at 5.47 (p-value = 0.0102), 4.33 (p-value = 0.0236), and 4.95 (p-value = 0.0159) fold greater risk of BC19 as compared to their peers, respectively. Importantly, every five-fold increase in viral neutralization capacities (by live-virus-based assays) was significantly associated with ~4-fold reduction in the risk occurrence of breakthrough COVID-19 (p-value = 0.045). Vaccine boosting remarkably increased these viral neutralization capacities by 16.22-fold (p- value = 0.0005), supporting the importance of the BNT162b2 booster in efforts to control the incursion of future variants into the population at large. Strikingly, BC19 cases exhibited a delayed/absent antibody response to the N protein, suggesting limited exposure to the virus. Since antibodies against N protein are widely used to evaluate the extent of virus spread in communities, our finding has important implications on the utility of existing serological diagnostic and surveillance for COVID-19.
Keywords: COVID-19 breakthrough; SARS-CoV-2; and N protein; booster.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


Similar articles
-
Vaccine-Induced Antibody Responses against SARS-CoV-2 Variants-Of-Concern Six Months after the BNT162b2 COVID-19 mRNA Vaccination.Microbiol Spectr. 2022 Apr 27;10(2):e0225221. doi: 10.1128/spectrum.02252-21. Epub 2022 Mar 9. Microbiol Spectr. 2022. PMID: 35262410 Free PMC article.
-
Postvaccination infections among staff of a tertiary care hospital after vaccination with severe acute respiratory syndrome coronavirus 2 vector and mRNA-based vaccines.Clin Microbiol Infect. 2022 Apr;28(4):596-601. doi: 10.1016/j.cmi.2021.11.023. Epub 2021 Dec 13. Clin Microbiol Infect. 2022. PMID: 34915073 Free PMC article.
-
BNT162b2 Booster Vaccination Elicits Cross-Reactive Immunity Against SARS-CoV-2 Variants B.1.1.529 and B.1.617.2 in Convalescents of All Ages.Front Immunol. 2022 Jun 20;13:920210. doi: 10.3389/fimmu.2022.920210. eCollection 2022. Front Immunol. 2022. PMID: 35795665 Free PMC article.
-
Virological and serological kinetics of SARS-CoV-2 Delta variant vaccine breakthrough infections: a multicentre cohort study.Clin Microbiol Infect. 2022 Apr;28(4):612.e1-612.e7. doi: 10.1016/j.cmi.2021.11.010. Epub 2021 Nov 23. Clin Microbiol Infect. 2022. PMID: 34826623 Free PMC article.
-
Differences in SARS-CoV-2-Specific Antibody Responses After the First, Second, and Third Doses of BNT162b2 in Naïve and Previously Infected Individuals: A 1-Year Observational Study in Healthcare Professionals.Front Immunol. 2022 May 27;13:876533. doi: 10.3389/fimmu.2022.876533. eCollection 2022. Front Immunol. 2022. PMID: 35711413 Free PMC article.
Cited by
-
Predicting severe COVID-19 using readily available admission indicators: SpO2/FiO2 ratio, comorbidity index, and gender.Exp Biol Med (Maywood). 2024 Nov 20;249:10193. doi: 10.3389/ebm.2024.10193. eCollection 2024. Exp Biol Med (Maywood). 2024. PMID: 39633683 Free PMC article.
References
-
- Centers for Disease Control and Prevention (CDC). COVID-19 vaccine breakthrough case investigation and reporting. Atlanta, GA: CDC, 2021
-
- Centers for Disease Control and Prevention (CDC). Interpretive summary for September 10, 2021: COVID data tracker weekly review. Atlanta, GA: CDC, 2022
-
- Centers for Disease Control and Prevention (CDC). COVID-19 vaccinations in the United States: COVID data tracker. Atlanta, GA: CDC, 2021
-
- Singer N. COVID-19 vaccine breakthrough cases: data from the States. KFF, 30 July 2021. https://www.kff.org/policy-watch/covid-19-vaccine-breakthrough-cases-dat...
-
- Website LDoH. LDH, Governor urge all individuals in Louisiana to mask up indoors against “dangerous and dominant” Delta variant. In: Health Do (ed.) LDH Website: LDH, 2021. https://ldh.la.gov/news/6257
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

