Distinct inflammation-related proteins associated with T cell immune recovery during chronic HIV-1 infection
- PMID: 36408648
- PMCID: PMC9769146
- DOI: 10.1080/22221751.2022.2150566
Distinct inflammation-related proteins associated with T cell immune recovery during chronic HIV-1 infection
Abstract
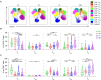
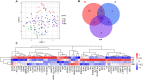
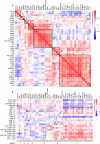
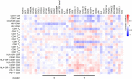
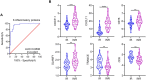
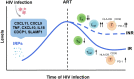
Chronic inflammation and T cell dysregulation persist in individuals infected with human immunodeficiency virus type 1 (HIV-1), even after successful antiretroviral treatment. The mechanism involved is not fully understood. Here, we used Olink proteomics to comprehensively analyze the aberrant inflammation-related proteins (IRPs) in chronic HIV-1-infected individuals, including in 24 treatment-naïve individuals, 33 immunological responders, and 38 immunological non-responders. T cell dysfunction was evaluated as T cell exhaustion, activation, and differentiation using flow cytometry. We identified a cluster of IRPs (cluster 7), including CXCL11, CXCL9, TNF, CXCL10, and IL18, which was closely associated with T cell dysregulation during chronic HIV-1 infection. Interestingly, IRPs in cluster 5, including ST1A1, CASP8, SIRT2, AXIN1, STAMBP, CD40, and IL7, were negatively correlated with the HIV-1 reservoir size. We also identified a combination of CDCP1, CXCL11, CST5, SLAMF1, TRANCE, and CD5, which may be useful for distinguishing immunological responders and immunological non-responders. In conclusion, the distinct inflammatory milieu is closely associated with immune restoration of T cells, and our results provide insight into immune dysregulation during chronic HIV-1 infection.
Keywords: CXCL10; CXCL11; CXCL9; HIV-1; HIV-1 reservoir; Inflammation; T cell dysregulation.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
