Treatment-related adverse events of antibody-drug conjugates in clinical trials: A systematic review and meta-analysis
- PMID: 36408673
- PMCID: PMC10099922
- DOI: 10.1002/cncr.34507
Treatment-related adverse events of antibody-drug conjugates in clinical trials: A systematic review and meta-analysis
Abstract
Background: Antibody-drug conjugates (ADCs) have complex molecular structures and have been tested in numerous clinical trials. Therefore, understanding the mechanisms of their toxicity when applied in medical practice is of high importance.
Methods: In a systematic review and meta-analysis of data gathered from different scientific databases (PubMed, Embase, Cochrane, and Web of Science) between January 1, 2000, and June 7, 2022, the authors applied a random-effects model with logit transformation and evaluated the heterogeneity between studies using I2 statistics. The primary outcome was the incidence and 95% confidence interval (CI) for all-grade and grade ≥3 treatment-related adverse events and differences between different drugs, molecular structures, and cancer types.
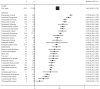
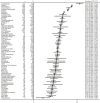
Results: In total, 2511 records were identified that included 169 clinical trials involving 22,492 patients. The overall incidence of treatment-related adverse events was 91.2% (95% CI, 90.7%-91.7%; I2 = 95.9%) for all-grade adverse events and 46.1% (95% CI, 45.2%-47.0%; I2 = 96.3%) for grade ≥3 adverse events. The most common all-grade adverse events were lymphopenia (53.0%; 95% CI, 48.7%-57.3%), nausea (44.1%; 95% CI, 43.2%-44.9%), neutropenia (43.7%; 95% CI, 42.6%-44.9%), blurred vision (40.5%; 95% CI, 37.4%-43.6%), and peripheral neuropathy (39.6%; 95% CI, 38.2%-41.1%); and the most common grade ≥3 adverse events were neutropenia (31.2%; 95% CI, 30.2%-32.3%), hypoesthesia (23.3%; 95% CI, 10.6%-35.9%), thrombocytopenia (22.6%; 95% CI, 21.3%-23.9%), febrile neutropenia (21.2%; 95% CI, 19.3%-23.1%), and lymphopenia (21.0%; 95% CI, 18.2%-23.7%).
Conclusions: Different ADCs appear to affect various treatment-related adverse events and provide comprehensive data on treatment-related adverse events for ADCs. The current results provide an important reference for clinicians and patients on how to care for toxicities from ADCs in clinical practice.
Lay summary: Unique anticancer drugs called antibody-drug conjugates (ADCs) have made significant progress in oncology in recent years because of their great success, and they are rapidly being used in the clinic as well as in hundreds of ongoing trials exploring their further use. The occurrence of serious side effects (adverse events) related to the receipt of ADCs was studied using data from 169 clinical trials involving 22,492 patients to determine the treatment-related causes of higher toxicity and adverse events in patients who receive ADCs, because these data are crucial for informing physicians how to safely treat patients using ADCs. The results indicate that different ADCs appear to affect various adverse events related to their use, providing comprehensive data on these ADCs that provide an important reference for clinicians and patients on how to care for toxicities from ADCs in clinical practice.
Keywords: antibody-drug conjugates (ADCs); clinical trials; monoclonal antibody (MoAb); oncology; payload; treatment-related adverse events.
© 2022 The Authors. Cancer published by Wiley Periodicals LLC on behalf of American Cancer Society.
Conflict of interest statement
The authors made no disclosures.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

