Disrupted T-tubular network accounts for asynchronous calcium release in MTM1-deficient skeletal muscle
- PMID: 36408764
- PMCID: PMC10107287
- DOI: 10.1113/JP283650
Disrupted T-tubular network accounts for asynchronous calcium release in MTM1-deficient skeletal muscle
Abstract
In mammalian skeletal muscle, the propagation of surface membrane depolarization into the interior of the muscle fibre along the transverse (T) tubular network is essential for the synchronized release of calcium from the sarcoplasmic reticulum (SR) via ryanodine receptors (RyRs) in response to the conformational change in the voltage-sensor dihydropyridine receptors. Deficiency in 3-phosphoinositide phosphatase myotubularin (MTM1) has been reported to disrupt T-tubules, resulting in impaired SR calcium release. Here confocal calcium transients recorded in muscle fibres of MTM1-deficient mice were compared with the results from a model where propagation of the depolarization along the T-tubules was modelled mathematically with disruptions in the network assumed to modify the access and transmembrane resistance as well as the capacitance. If, in simulations, T-tubules were assumed to be partially or completely inaccessible to the depolarization and RyRs at these points to be prime for calcium-induced calcium release, all the features of measured SR calcium release could be reproduced. We conclude that the inappropriate propagation of the depolarization into the fibre interior is the initial critical cause of severely impaired SR calcium release in MTM1 deficiency, while the Ca2+ -triggered opening of RyRs provides an alleviating support to the diseased process. KEY POINTS: Myotubular myopathy is a fatal disease due to genetic deficiency in the phosphoinositide phosphatase MTM1. Although the causes are known and corresponding gene therapy strategies are being developed, there is no mechanistic understanding of the disease-associated muscle function failure. Resolving this issue is of primary interest not only for a fundamental understanding of how MTM1 is critical for healthy muscle function, but also for establishing the related cellular mechanisms most primarily or stringently affected by the disease, which are thus of potential interest as therapy targets. The mathematical modelling approach used in the present work proves that the disease-associated alteration of the plasma membrane invagination network is sufficient to explain the dysfunctions of excitation-contraction coupling, providing the first integrated quantitative framework that explains the associated contraction failure.
Keywords: MTM1; T-tubule; calcium release; ryanodine receptor; sarcoplasmic reticulum.
© 2022 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
The authors declare no conflict of financial and non‐financial competing interests on behalf of all authors.
Figures
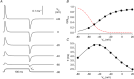



 ): N‐terminal; around amino acids 1873−1903. Activating site (
): N‐terminal; around amino acids 1873−1903. Activating site ( ): C‐terminal; amino acids 4007−5037. B and C, gating schemes of RyR for activation and inactivation with calcium. B, four‐state model of channel activation. C, four‐state model of channel inactivation. Note: the channel is open if A(ctivated) and N(ot‐inactivated). [Colour figure can be viewed at
): C‐terminal; amino acids 4007−5037. B and C, gating schemes of RyR for activation and inactivation with calcium. B, four‐state model of channel activation. C, four‐state model of channel inactivation. Note: the channel is open if A(ctivated) and N(ot‐inactivated). [Colour figure can be viewed at 







Similar articles
-
Ca2+-induced sarcoplasmic reticulum Ca2+ release in myotubularin-deficient muscle fibers.Cell Calcium. 2019 Jun;80:91-100. doi: 10.1016/j.ceca.2019.04.004. Epub 2019 Apr 10. Cell Calcium. 2019. PMID: 30999217
-
Phosphatidylinositol 3-kinase inhibition restores Ca2+ release defects and prolongs survival in myotubularin-deficient mice.Proc Natl Acad Sci U S A. 2016 Dec 13;113(50):14432-14437. doi: 10.1073/pnas.1604099113. Epub 2016 Nov 28. Proc Natl Acad Sci U S A. 2016. PMID: 27911767 Free PMC article.
-
Preserved Ca2+ handling and excitation-contraction coupling in muscle fibres from diet-induced obese mice.Diabetologia. 2020 Nov;63(11):2471-2481. doi: 10.1007/s00125-020-05256-8. Epub 2020 Aug 25. Diabetologia. 2020. PMID: 32840676
-
Organization of junctional sarcoplasmic reticulum proteins in skeletal muscle fibers.J Muscle Res Cell Motil. 2015 Dec;36(6):501-15. doi: 10.1007/s10974-015-9421-5. Epub 2015 Sep 15. J Muscle Res Cell Motil. 2015. PMID: 26374336 Review.
-
Molecular aspects of the excitation-contraction coupling in skeletal muscle.Jpn J Physiol. 1999 Aug;49(4):325-33. doi: 10.2170/jjphysiol.49.325. Jpn J Physiol. 1999. PMID: 10529492 Review.
Cited by
-
Reduced voltage-activated Ca2+ release flux in muscle fibers from a rat model of Duchenne dystrophy.J Gen Physiol. 2025 Mar 3;157(2):e202413588. doi: 10.1085/jgp.202413588. Epub 2024 Dec 24. J Gen Physiol. 2025. PMID: 39718509
References
-
- Al‐Qusairi, L. , Weiss, N. , Toussaint, A. , Berbey, C. , Messaddeq, N. , Kretz, C. , Sanoudou, D. , Beggs, A. H. , Allard, B. , Mandel, J. L. , Laporte, J. , Jacquemond, V. , & Buj‐Bello, A. (2009). T‐tubule disorganization and defective excitation‐contraction coupling in muscle fibres lacking myotubularin lipid phosphatase. Proceedings National Academy of Science USA, 106(44), 18763–18768. - PMC - PubMed
-
- Annoussamy, M. , Lilien, C. , Gidaro, T. , Gargaun, E. , Chê, V. , Schara, U. , Gangfuß, A. , D'Amico, A. , Dowling, J. J. , Darras, B. T. , Daron, A. , Hernandez, A. , de Lattre, C. , Arnal, J. M. , Mayer, M. , Cuisset, J. M. , Vuillerot, C. , Fontaine, S. , Bellance, R. , …, Servais, L. (2019). X‐linked myotubular myopathy: A prospective international natural history study. Neurology, 92(16), e1852‐e1867. - PMC - PubMed
-
- Bodnar, D. , Geyer, N. , Ruzsnavszky, O. , Olah, T. , Hegyi, B. , Sztretye, M. , Fodor, J. , Dienes, B. , Balogh, A. , Papp, Z. , Szabo, L. , Muller, G. , Csernoch, L. , & Szentesi, P. (2014). Hypermuscular mice with mutation in the myostatin gene display altered calcium signalling. The Journal of Physiology, 592(6), 1353–1365. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

