Composition, organization and mechanisms of the transition zone, a gate for the cilium
- PMID: 36408840
- PMCID: PMC9724682
- DOI: 10.15252/embr.202255420
Composition, organization and mechanisms of the transition zone, a gate for the cilium
Abstract
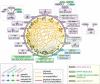
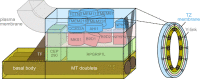
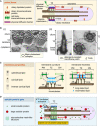
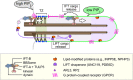
The cilium evolved to provide the ancestral eukaryote with the ability to move and sense its environment. Acquiring these functions required the compartmentalization of a dynein-based motility apparatus and signaling proteins within a discrete subcellular organelle contiguous with the cytosol. Here, we explore the potential molecular mechanisms for how the proximal-most region of the cilium, termed transition zone (TZ), acts as a diffusion barrier for both membrane and soluble proteins and helps to ensure ciliary autonomy and homeostasis. These include a unique complement and spatial organization of proteins that span from the microtubule-based axoneme to the ciliary membrane; a protein picket fence; a specialized lipid microdomain; differential membrane curvature and thickness; and lastly, a size-selective molecular sieve. In addition, the TZ must be permissive for, and functionally integrates with, ciliary trafficking systems (including intraflagellar transport) that cross the barrier and make the ciliary compartment dynamic. The quest to understand the TZ continues and promises to not only illuminate essential aspects of human cell signaling, physiology, and development, but also to unravel how TZ dysfunction contributes to ciliopathies that affect multiple organ systems, including eyes, kidney, and brain.
Keywords: cilia; ciliary gate; ciliary trafficking; ciliopathies; transition zone.
© 2022 The Authors.
Figures

References
-
- Andersen OS, Koeppe RE (2007) Bilayer thickness and membrane protein function: an energetic perspective. Annu Rev Biophys Biomol Struct 36: 107–130 - PubMed
-
- Arima T, Shibata Y, Yamamoto T (1984) A deep‐etching study of the guinea pig tracheal cilium with special reference to the ciliary transitional region. J Ultrastruct Res 89: 34–41 - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

