Counterion Tuning of Near-Infrared Organic Salts Dictates Phototoxicity to Inhibit Tumor Growth
- PMID: 36408853
- PMCID: PMC9743086
- DOI: 10.1021/acsami.2c16252
Counterion Tuning of Near-Infrared Organic Salts Dictates Phototoxicity to Inhibit Tumor Growth
Abstract
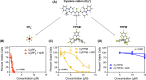
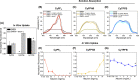
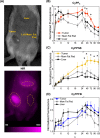
Photodynamic therapy (PDT) has the potential to improve cancer treatment by providing dual selectivity through the use of both photoactive agent and light, with the goal of minimal harmful effects from either the agent or light alone. However, current PDT is limited by insufficient photosensitizers (PSs) that can suffer from low tissue penetration, insufficient phototoxicity (toxicity with light irradiation), or undesirable cytotoxicity (toxicity without light irradiation). Recently, we reported a platform for decoupling optical and electronic properties with counterions that modulate frontier molecular orbital levels of a photoactive ion. Here, we demonstrate the utility of this platform in vivo by pairing near-infrared (NIR) photoactive heptamethine cyanine cation (Cy+), which has enhanced optical properties for deep tissue penetration, with counterions that make it cytotoxic, phototoxic, or nontoxic in a mouse model of breast cancer. We find that pairing Cy+ with weakly coordinating anion FPhB- results in a selectively phototoxic PS (CyFPhB) that stops tumor growth in vivo with minimal side effects. This work provides proof of concept that our counterion pairing platform can be used to generate improved cancer PSs that are selectively phototoxic to tumors and nontoxic to normal healthy tissues.
Keywords: heptamethine cyanine; metastatic breast cancer; near-infrared; photodynamic therapy; photosensitizer.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Next-Generation Photosensitizers: Cyanine-Carborane Salts for Superior Photodynamic Therapy of Metastatic Cancer.Angew Chem Int Ed Engl. 2025 Feb 24;64(9):e202419759. doi: 10.1002/anie.202419759. Epub 2025 Jan 31. Angew Chem Int Ed Engl. 2025. PMID: 39841576 Free PMC article.
-
Counterion influence on near-infrared-II heptamethine cyanine salts for photothermal therapy.Bioorg Chem. 2024 Apr;145:107206. doi: 10.1016/j.bioorg.2024.107206. Epub 2024 Feb 13. Bioorg Chem. 2024. PMID: 38367428
-
Modulating cellular cytotoxicity and phototoxicity of fluorescent organic salts through counterion pairing.Sci Rep. 2019 Oct 25;9(1):15288. doi: 10.1038/s41598-019-51593-z. Sci Rep. 2019. PMID: 31653966 Free PMC article.
-
Potential of Cyanine Derived Dyes in Photodynamic Therapy.Pharmaceutics. 2021 May 31;13(6):818. doi: 10.3390/pharmaceutics13060818. Pharmaceutics. 2021. PMID: 34072719 Free PMC article. Review.
-
Critical Overview of the Use of Ru(II) Polypyridyl Complexes as Photosensitizers in One-Photon and Two-Photon Photodynamic Therapy.Acc Chem Res. 2017 Nov 21;50(11):2727-2736. doi: 10.1021/acs.accounts.7b00180. Epub 2017 Oct 23. Acc Chem Res. 2017. PMID: 29058879 Review.
Cited by
-
Synthesis of Near-Infrared-Absorbing Anionic Heptamethine Cyanine Dyes with Trifluoromethyl Groups.Molecules. 2023 Jun 8;28(12):4650. doi: 10.3390/molecules28124650. Molecules. 2023. PMID: 37375210 Free PMC article.
-
Next-Generation Photosensitizers: Cyanine-Carborane Salts for Superior Photodynamic Therapy of Metastatic Cancer.Angew Chem Int Ed Engl. 2025 Feb 24;64(9):e202419759. doi: 10.1002/anie.202419759. Epub 2025 Jan 31. Angew Chem Int Ed Engl. 2025. PMID: 39841576 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

