Undesired impact of iron supplement on MRI assessment of post-treatment glioblastoma
- PMID: 36408899
- PMCID: PMC9830595
- DOI: 10.2217/cns-2021-0018
Undesired impact of iron supplement on MRI assessment of post-treatment glioblastoma
Abstract
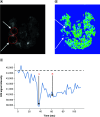
Glioblastoma (GBM) is the most common malignant adult brain and has a poor prognosis. Routine post-treatment MRI evaluations are required to assess treatment response and disease progression. We present a case of an 83-year-old female who underwent MRI assessment of post-treatment GBM after intravenous iron replacement therapy, ferumoxytol. The brain MRI revealed unintended alteration of MRI signal characteristics from the iron containing agent which confounded diagnostic interpretation and subsequently, the treatment planning. Ferumoxytol injection prior to contrast enhanced MRI must be screened in post-treatment GBM patients to accurately evaluate tumor activity.
Keywords: GBM; adverse events; brain tumor; ferumoxytol; imaging.
Figures


References
-
- Clarke JL, Chang S. Pseudoprogression and pseudoresponse: challenges in brain tumor imaging. Curr. Neurol. Neurosci. Rep. 9(3), 241–246 (2009). - PubMed
-
- Cha J, Kim ST, Kim HJ et al. Differentiation of tumor progression from pseudoprogression in patients with posttreatment glioblastoma using multiparametric histogram analysis. Am. J. Neuroradiol. 35(7), 1309–1317 (2014). - PMC - PubMed
-
•• Discusses the usefulness of multiparametric histogram analysis of post-treatment glioblastoma in predicting true tumor progression.
-
- Suh CH, Kim HS, Jung SC, Choi CG, Kim SJ. Multiparametric MRI as a potential surrogate endpoint for decision-making in early treatment response following concurrent chemoradiotherapy in patients with newly diagnosed glioblastoma: a systematic review and meta-analysis. Eur. Radiol. 28(6), 2628–2638 (2018). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
