Relative contributions of endogenous and exogenous formaldehyde to formation of deoxyguanosine monoadducts and DNA-protein crosslink adducts of DNA in rat nasal mucosa
- PMID: 36409013
- PMCID: PMC9887723
- DOI: 10.1093/toxsci/kfac119
Relative contributions of endogenous and exogenous formaldehyde to formation of deoxyguanosine monoadducts and DNA-protein crosslink adducts of DNA in rat nasal mucosa
Abstract
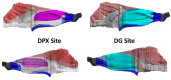
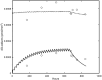
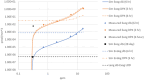
Understanding the dose-response for formaldehyde-induced nasal cancer in rats is complicated by (1) the uneven distribution of inhaled formaldehyde across the interior surface of the nasal cavity and, (2) the presence of endogenous formaldehyde (endoF) in the nasal mucosa. In this work, we used computational fluid dynamics (CFD) modeling to predict flux of inhaled (exogenous) formaldehyde (exogF) from air into tissue at the specific locations where DNA adducts were measured. Experimental work has identified DNA-protein crosslink (DPX) adducts due to exogF and deoxyguanosine (DG) adducts due to both exogF and endoF. These adducts can be considered biomarkers of exposure for effects of endoF and exogF on DNA that may be part of the mechanism of tumor formation. We describe a computational model linking CFD-predicted flux of formaldehyde from air into tissue, and the intracellular production of endoF, with the formation of DPX and DG adducts. We assumed that, like exogF, endoF can produce DPX. The model accurately reproduces exogDPX, exogDG, and endoDG data after inhalation from 0.7 to 15 ppm. The dose-dependent concentrations of exogDPX and exogDG are predicted to exceed the concentrations of their endogenous counterparts at about 2 and 6 ppm exogF, respectively. At all concentrations examined, the concentrations of endoDPX and exogDPX were predicted to be at least 10-fold higher than that of their DG counterparts. The modeled dose-dependent concentrations of these adducts are suitable to be used together with data on the dose-dependence of cell proliferation to conduct quantitative modeling of formaldehyde-induced rat nasal carcinogenicity.
Keywords: BBDR; CFD; DNA adducts; dosimetry; endogenous; formaldehyde; nasal.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Society of Toxicology.
Figures




Similar articles
-
Updating the biologically based dose-response model for the nasal carcinogenicity of inhaled formaldehyde in the F344 rat.Toxicol Sci. 2023 May 12;193(1):1-17. doi: 10.1093/toxsci/kfad028. Toxicol Sci. 2023. PMID: 36912747 Free PMC article.
-
Effects of endogenous formaldehyde in nasal tissues on inhaled formaldehyde dosimetry predictions in the rat, monkey, and human nasal passages.Toxicol Sci. 2014 Apr;138(2):412-24. doi: 10.1093/toxsci/kft333. Epub 2014 Jan 2. Toxicol Sci. 2014. PMID: 24385418
-
Distribution of DNA adducts caused by inhaled formaldehyde is consistent with induction of nasal carcinoma but not leukemia.Toxicol Sci. 2010 Aug;116(2):441-51. doi: 10.1093/toxsci/kfq061. Epub 2010 Feb 22. Toxicol Sci. 2010. PMID: 20176625 Free PMC article.
-
The implausibility of leukemia induction by formaldehyde: a critical review of the biological evidence on distant-site toxicity.Regul Toxicol Pharmacol. 2004 Oct;40(2):92-106. doi: 10.1016/j.yrtph.2004.05.001. Regul Toxicol Pharmacol. 2004. PMID: 15450713 Review.
-
Dosimetry, toxicity and carcinogenicity of inspired acetaldehyde in the rat.Mutat Res. 1997 Oct 31;380(1-2):113-24. doi: 10.1016/s0027-5107(97)00130-9. Mutat Res. 1997. PMID: 9385393 Review.
Cited by
-
Repair of genomic interstrand crosslinks.DNA Repair (Amst). 2024 Sep;141:103739. doi: 10.1016/j.dnarep.2024.103739. Epub 2024 Jul 30. DNA Repair (Amst). 2024. PMID: 39106540 Free PMC article. Review.
-
New approach methodologies (NAMs) for the in vitro assessment of cleaning products for respiratory irritation: workshop report.Front Toxicol. 2024 Oct 8;6:1431790. doi: 10.3389/ftox.2024.1431790. eCollection 2024. Front Toxicol. 2024. PMID: 39439531 Free PMC article. Review.
-
Updating the biologically based dose-response model for the nasal carcinogenicity of inhaled formaldehyde in the F344 rat.Toxicol Sci. 2023 May 12;193(1):1-17. doi: 10.1093/toxsci/kfad028. Toxicol Sci. 2023. PMID: 36912747 Free PMC article.
References
-
- Andersen M. E., Gentry P. R., Swenberg J. A., Mundt K. A., White K. W., Thompson C., Bus J., Sherman J. H., Greim H., Bolt H., et al. (2019). Considerations for refining the risk assessment process for formaldehyde: Results from an interdisciplinary workshop. Regul. Toxicol. Pharmacol. 106, 210–223. - PubMed
-
- Beane Freeman L. E., Blair A., Lubin J. H., Stewart P. A., Hayes R. B., Hoover R. N., Hauptmann M. (2013). Mortality from solid tumors among workers in formaldehyde industries: An update of the NCI cohort. Am. J. Ind. Med. 56, 1015–1026. - PubMed
-
- Bergstresser P. R., Pariser R. J., Taylor J. R. (1978). Counting and sizing of epidermal cells in normal human skin. J. Invest. Dermatol. 70, 280–284. - PubMed
-
- Blair A., Stewert P., O’Berg M., Gaffey W. R., Walrath J., Ward J., Cubir D. (1986). Mortality among industrial workers exposed to formaldehyde [see comments]. J. Natl. Cancer Inst. 76, 1071–1084. - PubMed
-
- Campbell J. L. Jr., Gentry P. R., Clewell H. J. III, Andersen M. E. (2020). A kinetic analysis of DNA-deoxy-guanine adducts in the nasal epithelium produced by inhaled formaldehyde in rats—Assessing contributions to adduct production from both endogenous and exogenous sources of formaldehyde. Toxicol. Sci. 177, 325–333. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

