Streptococcus agalactiae npx Is Required for Survival in Human Placental Macrophages and Full Virulence in a Model of Ascending Vaginal Infection during Pregnancy
- PMID: 36409087
- PMCID: PMC9765263
- DOI: 10.1128/mbio.02870-22
Streptococcus agalactiae npx Is Required for Survival in Human Placental Macrophages and Full Virulence in a Model of Ascending Vaginal Infection during Pregnancy
Abstract
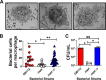
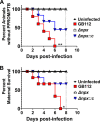
Streptococcus agalactiae, also known as group B Streptococcus (GBS), is a Gram-positive encapsulated bacterium that colonizes the gastrointestinal tract of 30 to 50% of humans. GBS causes invasive infection during pregnancy that can lead to chorioamnionitis, funisitis, preterm prelabor rupture of membranes (PPROM), preterm birth, neonatal sepsis, and maternal and fetal demise. Upon infecting the host, GBS encounters sentinel innate immune cells, such as macrophages, within reproductive tissues. Once phagocytosed by macrophages, GBS upregulates the expression of the gene npx, which encodes an NADH peroxidase. GBS mutants with an npx deletion (Δnpx) are exquisitely sensitive to reactive oxygen stress. Furthermore, we have shown that npx is required for GBS survival in both THP-1 and placental macrophages. In an in vivo murine model of ascending GBS vaginal infection during pregnancy, npx is required for invading reproductive tissues and is critical for inducing disease progression, including PPROM and preterm birth. Reproductive tissue cytokine production was also significantly diminished in Δnpx mutant-infected animals compared to that in animals infected with wild-type (WT) GBS. Complementation in trans reversed this phenotype, indicating that npx is critical for GBS survival and the initiation of proinflammatory signaling in the gravid host. IMPORTANCE This study sheds new light on the way that group B Streptococcus (GBS) defends itself against oxidative stress in the infected host. The enzyme encoded by the GBS gene npx is an NADH peroxidase that, our study reveals, provides defense against macrophage-derived reactive oxygen stress and facilitates infections of the uterus during pregnancy. This enzyme could represent a tractable target for future treatment strategies against invasive GBS infections.
Keywords: ROS; Streptococcus; infection; innate immunity; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Streptococcus agalactiae Induces Placental Macrophages To Release Extracellular Traps Loaded with Tissue Remodeling Enzymes via an Oxidative Burst-Dependent Mechanism.mBio. 2018 Nov 20;9(6):e02084-18. doi: 10.1128/mBio.02084-18. mBio. 2018. PMID: 30459195 Free PMC article.
-
The Group B Streptococcal Adhesin BspC Interacts with Host Cytokeratin 19 To Promote Colonization of the Female Reproductive Tract.mBio. 2022 Oct 26;13(5):e0178122. doi: 10.1128/mbio.01781-22. Epub 2022 Sep 7. mBio. 2022. PMID: 36069447 Free PMC article.
-
Bacterial Hyaluronidase Promotes Ascending GBS Infection and Preterm Birth.mBio. 2016 Jun 28;7(3):e00781-16. doi: 10.1128/mBio.00781-16. mBio. 2016. PMID: 27353757 Free PMC article.
-
Mechanisms of group B Streptococcus-mediated preterm birth: lessons learnt from animal models.Reprod Fertil. 2022 Jun 7;3(3):R109-R120. doi: 10.1530/RAF-21-0105. eCollection 2022 Jul 1. Reprod Fertil. 2022. PMID: 35794927 Free PMC article. Review.
-
The Double Life of Group B Streptococcus: Asymptomatic Colonizer and Potent Pathogen.J Mol Biol. 2019 Jul 26;431(16):2914-2931. doi: 10.1016/j.jmb.2019.01.035. Epub 2019 Jan 31. J Mol Biol. 2019. PMID: 30711542 Free PMC article. Review.
Cited by
-
Environmental Toxicant Exposure Paralyzes Human Placental Macrophage Responses to Microbial Threat.ACS Infect Dis. 2023 Dec 8;9(12):2401-2408. doi: 10.1021/acsinfecdis.3c00490. Epub 2023 Nov 13. ACS Infect Dis. 2023. PMID: 37955242 Free PMC article.
-
The Utility of Human Milk Oligosaccharides against Group B Streptococcus Infections of Reproductive Tissues and Cognate Adverse Pregnancy Outcomes.ACS Cent Sci. 2023 Aug 9;9(9):1737-1749. doi: 10.1021/acscentsci.3c00101. eCollection 2023 Sep 27. ACS Cent Sci. 2023. PMID: 37780357 Free PMC article.
-
Evaluating impacts of the trichloroethylene metabolite S-(1,2-dichlorovyinyl)-L-cysteine on transcriptomic responses and cytokine release in a macrophage model: implications for pregnancy outcomes.J Immunotoxicol. 2025 Dec;22(1):2522041. doi: 10.1080/1547691X.2025.2522041. Epub 2025 Jul 3. J Immunotoxicol. 2025. PMID: 40607769
References
-
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, Munson ML, Centers for Disease Control and Prevention National Center for Health Statistics National Vital Statistics System . 2007. Births: final data for 2005. Natl Vital Stat Rep 56:1–103. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

