Long-Term Protection Elicited by an Inactivated Vaccine Supplemented with a Water-Based Adjuvant against Infectious Hematopoietic Necrosis Virus in Rainbow Trout (Oncorhynchus mykiss)
- PMID: 36409094
- PMCID: PMC9769665
- DOI: 10.1128/spectrum.03245-22
Long-Term Protection Elicited by an Inactivated Vaccine Supplemented with a Water-Based Adjuvant against Infectious Hematopoietic Necrosis Virus in Rainbow Trout (Oncorhynchus mykiss)
Abstract
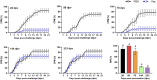
Previous inactivated vaccines against infectious hematopoietic necrosis (IHN) usually had a strong early immune protective effect but failed to provide long-term protection in rainbow trout (Oncorhynchus mykiss). To find a method for stabilizing the desired protective effect of IHN vaccines, we assessed the immune enhancement effect of four adjuvants on formaldehyde inactivated vaccine for IHN at 60 days postvaccination (dpv). The efficacy of a two-dose vaccination with the candidate adjuvant-formaldehyde inactivated vaccine for IHN was evaluated in terms of early protection and long-term protection (30 to 285 dpv). Neutralizing antibody titers were also measured at each time point. The Montanide GEL 02 PR (Gel 02) adjuvant significantly enhanced the immune protection provided by the IHN inactivated vaccine, whereas the immune-boosting effect of the other tested adjuvants lacked statistical significance. Both tested Gel 02-adjuvanted IHN inactivated vaccine dosages had a strong immune protection effect within 2 months postvaccination, with a relative percent of survival (RPS) of 89.01% to 100%, and the higher dosage provided complete protection at 204 dpv and a RPS of 60.79% on 285 dpv by reducing viral titers in rainbow trout. The neutralizing antibodies were observed only in vaccinated fish on 30 and 60 dpv. Through compatibility with an appropriate adjuvant, the highly immune protective effect of an IHN inactivated vaccine was prolonged from 60 dpv to at least 284 dpv; this novel adjuvant-IHN inactivated vaccine has promise as a commercial vaccine that provides the best available and longest duration of protection against IHN to rainbow trout. IMPORTANCE Infectious hematopoietic necrosis virus (IHNV) is one of the most serious pathogens threatening the global salmon and trout industry. However, there is currently only one commercialized infectious hematopoietic necrosis (IHN) vaccine, and it is inadequate for solving the global IHN problem. In this study, a promising adjuvanted inactivated vaccine with long-term protection was developed and comprehensively studied. We confirmed the presence of a late antiviral response stage in vaccinated rainbow trout that lacked detectable neutralizing antibodies, which are commonly recognized to be responsible for long-term specific protection in mammals. These findings further our understanding of unique features of fish immune systems and could lead to improved prevention and control of fish diseases.
Keywords: adjuvant; inactivated vaccine; infectious hematopoietic necrosis virus; long-term protection; rainbow trout.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

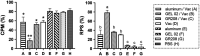



References
-
- Food and Agriculture Organization. 2020. FAO yearbook. Fishery and aquaculture statistics 2018. FAO, Rome, Italy. http://www.fao.org/documents/card/en/c/cb1213t. Retrieved 22 June 2021.
-
- Wolf K. 1988. Fish viruses and fish viral diseases. Cornell University Press, Ithaca, NY. https://www.oie.int/en/home.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

