Plasmodium falciparum Sexual Commitment Rate Variation among Clinical Isolates and Diverse Laboratory-Adapted Lines
- PMID: 36409095
- PMCID: PMC9769538
- DOI: 10.1128/spectrum.02234-22
Plasmodium falciparum Sexual Commitment Rate Variation among Clinical Isolates and Diverse Laboratory-Adapted Lines
Abstract
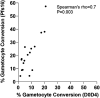
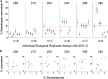
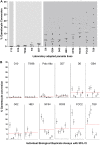
Asexual blood-stage malaria parasites must produce sexual progeny to infect mosquitoes. It is important to understand the scope and causes of intraspecific variation in sexual commitment rates, particularly for the major human parasite P. falciparum. First, two alternative assay methods of measuring sexual commitment were compared to test a genetically modified P. falciparum line with elevated commitment rates inducible by overexpression of GDV1. The methods yielded correlated measurements with higher sensitivity and precision being achieved by one employing detection of the early gametocyte differentiation marker Pfs16. Thus, this was used to survey a diverse range of parasite lines and test each in multiple biological replicate assays in a serum-free medium supplemented with Albumax. There were differences among six recent clinical isolates from Ghana in their mean rates of sexual commitment per cycle, ranging from 3.3% to 12.2%. Among 13 diverse long-term laboratory-adapted lines, mean sexual commitment rates for most ranged from 4.7% to 13.4%, a few had lower rates with means from 0.3 to 1.6%, and one with a nonfunctional ap2-g gene always showed zero commitment. Among a subset of lines tested for the effects of exogenous choline to suppress commitment, there were significant differences. As expected, there was no effect in a line that had lost the gdv1 gene and that had generally low commitment, whereas the others showed quantitatively variable but significant responses to choline, suggesting potential trait variation. The results indicated the value of performing multiple replicate assays for understanding the variation of this key reproductive trait that likely affects transmission. IMPORTANCE Only sexual-stage malaria parasites are transmitted from human blood to mosquitoes. Thus, it is vital to understand variations in sexual commitment rates because these may be modifiable or susceptible to blocking. Two different methods of commitment rate measurement were first compared, demonstrating higher sensitivity and precision by the detection of an early differentiation marker, which was subsequently used to survey diverse lines. Clinical isolates from Ghana showed significant variation in mean per-cycle commitment rates and variation among biological replicates. Laboratory-adapted lines of diverse origins had a wider range with most being within the range observed for the clinical isolates, while a minority consistently had lower or zero rates. There was quantitative variation in the effects when adding choline to suppress commitment, indicating differing responsiveness of parasites to this environmental modification. Performing multiple assay replicates and comparisons of diverse isolates was important to understand this trait and its potential effects on transmission.
Keywords: clinical isolates; differentiation; gametocyte; intraspecific variation; sexual development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- WHO. 2021. World Malaria Report 2021. Geneva.
-
- Barry A, Bradley J, Stone W, Guelbeogo MW, Lanke K, Ouedraogo A, Soulama I, Nebie I, Serme SS, Grignard L, Patterson C, Wu L, Briggs JJ, Janson O, Awandu SS, Ouedraogo M, Tarama CW, Kargougou D, Zongo S, Sirima SB, Marti M, Drakeley C, Tiono AB, Bousema T. 2021. Higher gametocyte production and mosquito infectivity in chronic compared to incident Plasmodium falciparum infections. Nat Commun 12:2443. doi:10.1038/s41467-021-22573-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

