Clinical Performance of Direct RT-PCR Testing of Raw Saliva for Detection of SARS-CoV-2 in Symptomatic and Asymptomatic Individuals
- PMID: 36409097
- PMCID: PMC9769602
- DOI: 10.1128/spectrum.02229-22
Clinical Performance of Direct RT-PCR Testing of Raw Saliva for Detection of SARS-CoV-2 in Symptomatic and Asymptomatic Individuals
Abstract
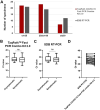
RT-PCR tests based on RNA extraction from nasopharyngeal swabs (NPS) are promoted as the "gold standard" for SARS-CoV-2 detection. However, the use of saliva samples offers noninvasive self-collection more suitable for high-throughput testing. This study evaluated performance of the TaqPath COVID-19 Fast PCR Combo kit 2.0 assay for detection of SARS-CoV-2 in raw saliva relative to a lab-developed direct RT-PCR test (SalivaDirect-based PCR, SDB-PCR) and an RT-PCR test based on RNA extraction from NPS. Saliva and NPS samples were collected from symptomatic and asymptomatic individuals (N = 615). Saliva samples were tested for SARS-CoV-2 using the TaqPath COVID-19 Fast PCR Combo kit 2.0 and the SDB-PCR, while NPS samples were tested by RT-PCR in RNA extracts according to the Irish national testing system. TaqPath COVID-19 Fast PCR Combo kit 2.0 detected SARS-CoV-2 in 52 saliva samples, of which 51 were also positive with the SDB-PCR. Compared to the NPS "gold standard" biospecimen method, 49 samples displayed concordant results, while three samples (35<Ct<37) were positive on raw saliva. Among the negative samples, 10 discordant cases were found with the TaqPath COVID-19 Fast PCR Combo kit 2.0 (PPA-83.05%; NPA-99.44%), compared to the RNA extraction-based NPS method, performing similarly to the SDB-PCR (PPA-84.75%; NPA-99.63%). The direct RT-PCR testing of saliva samples shows high concordance with the NPS extraction-based method for SARS-CoV-2 detection, and therefore provides a cost-effective and highly scalable system for high-throughput COVID-19 rapid-testing. IMPORTANCE The scale of the COVID-19 pandemic highlighted the need for viral diagnostic systems that are accurate and could be deployed at large population scales. Large-scale diagnostic or surveillance testing of large numbers of people requires collection of infected biological samples that is easy and rapid. Here, we demonstrate that raw saliva samples can be easily collected and tested by RT-PCR assays. Indeed, we find that direct testing of raw saliva by two different RT-PCR assays is as accurate (if not more accurate) than nasal swab-based RT-PCR testing. We present a cost-effective and highly scalable system for high-throughput COVID-19 rapid-testing.
Keywords: COVID-19; RT-PCR; SARS-CoV-2; asymptomatic; d; saliva; surveillance; symptomatic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Evaluation of self-collected nasal, urine, and saliva samples for molecular detection of SARS-CoV-2 using an EUA approved RT-PCR assay and a laboratory developed LAMP SARS-CoV-2 test.Immun Inflamm Dis. 2024 Jun;12(6):e1285. doi: 10.1002/iid3.1285. Immun Inflamm Dis. 2024. PMID: 38888444 Free PMC article.
-
Saliva as a testing specimen with or without pooling for SARS-CoV-2 detection by multiplex RT-PCR test.PLoS One. 2021 Feb 23;16(2):e0243183. doi: 10.1371/journal.pone.0243183. eCollection 2021. PLoS One. 2021. PMID: 33621263 Free PMC article.
-
Diagnostic Performance of Self-Collected Saliva Versus Nasopharyngeal Swab for the Molecular Detection of SARS-CoV-2 in the Clinical Setting.Microbiol Spectr. 2021 Dec 22;9(3):e0046821. doi: 10.1128/Spectrum.00468-21. Epub 2021 Nov 3. Microbiol Spectr. 2021. PMID: 34730436 Free PMC article.
-
Screening for SARS-CoV-2 by RT-PCR: Saliva or nasopharyngeal swab? Rapid review and meta-analysis.PLoS One. 2021 Jun 10;16(6):e0253007. doi: 10.1371/journal.pone.0253007. eCollection 2021. PLoS One. 2021. PMID: 34111196 Free PMC article.
-
Salivette, a relevant saliva sampling device for SARS-CoV-2 detection.J Oral Microbiol. 2021 Apr 30;13(1):1920226. doi: 10.1080/20002297.2021.1920226. J Oral Microbiol. 2021. PMID: 33986939 Free PMC article. Review.
Cited by
-
Assessment of the Effective Sensitivity of SARS-CoV-2 Sample Pooling Based on a Large-Scale Screening Experience: Retrospective Analysis.JMIR Public Health Surveill. 2024 Sep 24;10:e54503. doi: 10.2196/54503. JMIR Public Health Surveill. 2024. PMID: 39316785 Free PMC article.
-
Comparison of SARS-CoV-2 Detection in Nasopharyngeal Swab and Saliva Samples from Patients Infected with Omicron Variant.Int J Mol Sci. 2023 Mar 2;24(5):4847. doi: 10.3390/ijms24054847. Int J Mol Sci. 2023. PMID: 36902277 Free PMC article.
-
Saliva Is a Sensitive and Accessible Sample Both for SARS-CoV-2 Detection and for the Evaluation of Treatment Effectiveness in Follow-Up Studies.Viruses. 2024 Jun 27;16(7):1040. doi: 10.3390/v16071040. Viruses. 2024. PMID: 39066203 Free PMC article.
References
-
- Wyllie AL, Fournier J, Casanovas-Massana A, Campbell M, Tokuyama M, Vijayakumar P, Warren JL, Geng B, Muenker MC, Moore AJ, Vogels CBF, Petrone ME, Ott IM, Lu P, Venkataraman A, Lu-Culligan A, Klein J, Earnest R, Simonov M, Datta R, Handoko R, Naushad N, Sewanan LR, Valdez J, White EB, Lapidus S, Kalinich CC, Jiang X, Kim DJ, Kudo E, Linehan M, Mao T, Moriyama M, Oh JE, Park A, Silva J, Song E, Takahashi T, Taura M, Weizman O-E, Wong P, Yang Y, Bermejo S, Odio CD, Omer SB, Dela Cruz CS, Farhadian S, Martinello RA, Iwasaki A, Grubaugh ND, et al. 2020. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. N Engl J Med 383:1283–1286. doi:10.1056/NEJMc2016359. - DOI - PMC - PubMed
-
- Butler-Laporte G, Lawandi A, Schiller I, Yao M, Dendukuri N, McDonald EG, Lee TC. 2021. Comparison of saliva and nasopharyngeal swab nucleic acid amplification testing for detection of SARS-CoV-2: a systematic review and meta-analysis. JAMA Intern Med 181:353–360. doi:10.1001/jamainternmed.2020.8876. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

