Comparison of the BioFire Joint Infection Panel to 16S Ribosomal RNA Gene-Based Targeted Metagenomic Sequencing for Testing Synovial Fluid from Patients with Knee Arthroplasty Failure
- PMID: 36409108
- PMCID: PMC9769560
- DOI: 10.1128/jcm.01126-22
Comparison of the BioFire Joint Infection Panel to 16S Ribosomal RNA Gene-Based Targeted Metagenomic Sequencing for Testing Synovial Fluid from Patients with Knee Arthroplasty Failure
Abstract
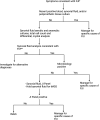
The diagnosis of periprosthetic joint infection (PJI) is challenging, often requiring multiple clinical specimens and diagnostic techniques, some with prolonged result turnaround times. Here, the diagnostic performance of the Investigational Use Only (IUO) BioFire Joint Infection (JI) Panel was compared to 16S rRNA gene-based targeted metagenomic sequencing (tMGS) applied to synovial fluid for PJI diagnosis. Sixty synovial fluid samples from knee arthroplasty failure archived at -80°C were tested. Infectious Diseases Society of America (IDSA) diagnostic criteria were used to classify PJI. For culture-positive PJI with pathogens targeted by the JI panel, JI panel sensitivity was 91% (21/23; 95% confidence interval [CI], 73 to 98%), and tMGS sensitivity was 96% (23/24; 95% CI, 80 to 99%) (P = 0.56). Overall sensitivities of the JI panel and tMGS for PJI diagnosis were 56% (24/43; 95% CI, 41 to 70%) and 93% (41/44; 95% CI, 82 to 98%), respectively (P < 0.001). JI panel and tMGS overall specificities were 100% (16/16; 95% CI, 81 to 100%) and 94% (15/16; 95% CI, 72 to 99%), respectively. While the clinical sensitivity of the JI panel was excellent for on-panel microorganisms, overall sensitivity for PJI diagnosis was low due to the absence of Staphylococcus epidermidis, a common causative pathogen of PJI, on the panel. A PJI diagnostic algorithm for the use of both molecular tests is proposed.
Keywords: PCR; next-generation sequencing; periprosthetic joint infection; rapid diagnostic.
Conflict of interest statement
The authors declare a conflict of interest. M.A.A., M.J.W., A.P.S., M.L.D., J.C.S., A.D.D., M.P.A., and K.E.G.-Q. have no conflicts of interests to declare. R.P. reports grants from ContraFect, TenNor Therapeutics Limited, and BioFire. R.P. is a consultant to Curetis, Next Gen Diagnostics, PathoQuest, Selux Diagnostics, 1928 Diagnostics, PhAST, Torus Biosystems, Day Zero Diagnostics, Mammoth Biosciences, and Qvella; monies are paid to Mayo Clinic. Mayo Clinic and R.P. have a relationship with Pathogenomix. R.P. has research supported by Adaptive Phage Therapeutics. Mayo Clinic has a royalty-bearing know-how agreement and equity in Adaptive Phage Therapeutics. R.P. is also a consultant to Netflix and CARB-X. In addition, R.P. has a patent on
Figures
References
-
- Atkins BL, Athanasou N, Deeks JJ, Crook DW, Simpson H, Peto TE, McLardy-Smith P, Berendt AR, The OSIRIS Collaborative Study Group . 1998. Prospective evaluation of criteria for microbiological diagnosis of prosthetic joint infection at revision arthroplasty. J Clin Microbiol 36:2932–2939. doi: 10.1128/JCM.36.10.2932-2939.1998. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

