Restriction of Influenza A Virus by SERINC5
- PMID: 36409124
- PMCID: PMC9765469
- DOI: 10.1128/mbio.02923-22
Restriction of Influenza A Virus by SERINC5
Abstract
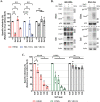
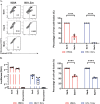
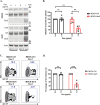
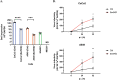
Serine incorporator 5 (Ser5), a transmembrane protein, has recently been identified as a host antiviral factor against human immunodeficiency virus (HIV)-1 and gammaretroviruses like murine leukemia viruses (MLVs). It is counteracted by HIV-1 Nef and MLV glycogag. We have investigated whether it has antiviral activity against influenza A virus (IAV), as well as retroviruses. Here, we demonstrated that Ser5 inhibited HIV-1-based pseudovirions bearing IAV hemagglutinin (HA); as expected, the Ser5 effect on this glycoprotein was antagonized by HIV-1 Nef protein. We found that Ser5 inhibited the virus-cell and cell-cell fusion of IAV, apparently by interacting with HA proteins. Most importantly, overexpressed and endogenous Ser5 inhibited infection by authentic IAV. Single-molecular fluorescent resonance energy transfer (smFRET) analysis further revealed that Ser5 both destabilized the pre-fusion conformation of IAV HA and inhibited the coiled-coil formation during membrane fusion. Ser5 is expressed in cultured small airway epithelial cells, as well as in immortal human cell lines. In summary, Ser5 is a host antiviral factor against IAV which acts by blocking HA-induced membrane fusion. IMPORTANCE SERINC5 (Ser5) is a cellular protein which has been found to interfere with the infectivity of HIV-1 and a number of other retroviruses. Virus particles produced in the presence of Ser5 are impaired in their ability to enter new host cells, but the mechanism of Ser5 action is not well understood. We now report that Ser5 also inhibits infectivity of Influenza A virus (IAV) and that it interferes with the conformational changes in IAV hemagglutinin protein involved in membrane fusion and virus entry. These findings indicate that the antiviral function of Ser5 extends to other viruses as well as retroviruses, and also provide some information on the molecular mechanism of its antiviral activity.
Keywords: SERINC5; hemagglutinin; influenza A virus; restriction; virus entry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ahi YS, Zhang S, Thappeta Y, Denman A, Feizpour A, Gummuluru S, Reinhard B, Muriaux D, Fivash MJ, Rein A. 2016. Functional interplay between murine leukemia virus glycogag, Serinc5, and surface glycoprotein governs virus entry, with opposite effects on gammaretroviral and ebolavirus glycoproteins. mBio 7:e01985-16. doi:10.1128/mBio.01985-16. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
