SARS-CoV-2 Anti-Spike IgG Antibody and ACE2 Receptor Binding Inhibition Levels among Breakthrough Stage Veteran Patients
- PMID: 36409132
- PMCID: PMC9769865
- DOI: 10.1128/spectrum.02747-22
SARS-CoV-2 Anti-Spike IgG Antibody and ACE2 Receptor Binding Inhibition Levels among Breakthrough Stage Veteran Patients
Abstract
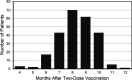
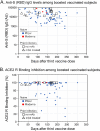
SARS-CoV-2 mRNA vaccines have been critical to curbing pandemic COVID-19; however, a major shortcoming has been the inability to assess levels of protection after vaccination. This study assessed serologic status of breakthrough infections in vaccinated patients at a Veterans Administration medical center from June through December 2021 during a SARS-CoV-2 delta variant wave. Breakthrough occurred mostly beyond 150 days after two-dose vaccination with a mean of 239 days. Anti-SARS-CoV-2 spike (S) IgG levels were low at 0 to 2 days postsymptoms but increased in subjects presenting thereafter. Population measurements of anti-S IgG and angiotensin converting enzyme-2 receptor (ACE2-R) binding inhibition among uninfected, vaccinated patients suggested immune decay occurred after 150 days with 62% having anti-S IgG levels at or below 1,000 AU comparable with breakthrough patients at 0 to 2 days postsymptom onset. In contrast, vaccination after resolved infection conferred robust enduring anti-S IgG levels (5,000 to >50,000 AU) with >90% ACE2-R binding inhibition. However, monoclonal antibody (MAb)-treated patients did not benefit from their prior infection suggesting impaired establishment of B cell memory. Analysis of boosted patients confirmed the benefit of a third vaccine dose with most having anti-S IgG levels above 5,000 AU with >90% ACE2-R binding inhibition, but a subset had levels <5,000 AU. Anti-S IgG levels >5,000 AU were associated with >90% ACE2-R binding inhibition and no documented breakthrough infections, whereas levels falling below 5,000 AU and approaching 1,000 AU were associated with breakthrough infections. Thus, quantitative antibody measurements may provide a means to guide vaccination intervals for the individual. IMPORTANCE Currently, clinicians have no guidance for the serologic assessment of SARS-Cov-2 postvaccination status regarding protection and risk of infection. Vaccination and boosters are administered blindly without evaluation of need or outcome at the individual level. The recent development of automated quantitative assays for anti-SARS-CoV-2 spike protein IgG antibodies permits accurate measurement of humoral immunity in standardized units. Clinical studies, such as reported here, will help establish protective antibody levels allowing identification and targeted management of poor vaccine responders and vaccinated subjects undergoing immune decay.
Keywords: ACE2 receptor; COVID-19; SARS-CoV-2; antibody assay; breakthrough infection; immunization; serology.
Conflict of interest statement
The authors declare a conflict of interest. Three authors, David J. Daghfal, John Prostko and Edwin Frias are employees of Abbott Laboratories. An Abbott test assay was used in this study. Their role was assay development and the testing of deidentified specimens. All other authors declare no conflict of interest.
Figures



References
-
- Almendro-Vázquez P, Laguna-Goya R, Ruiz-Ruigomez M, Utrero-Rico A, Lalueza A, Maestro de la Calle G, Delgado P, Perez-Ordoño L, Muro E, Vila J, Zamarron I, Moreno-Batanero M, Chivite-Lacaba M, Gil-Etayo FJ, Martín-Higuera C, Meléndez-Carmona M, Lumbreras C, Arellano I, Alarcon B, Allende LM, Aguado JM, Paz-Artal E. 2021. Longitudinal dynamics of SARS-CoV-2-specific cellular and humoral immunity after natural infection or BNT162b2 vaccination. PLoS Pathog 17:e1010211. doi: 10.1371/journal.ppat.1010211. - DOI - PMC - PubMed
-
- Chivu-Economescu M, Bleotu C, Grancea C, Chiriac D, Botezatu A, Iancu IV, Pitica I, Necula LG, Neagu A, Matei L, Dragu D, Sultana C, Radu EL, Nastasie A, Voicu O, Ataman M, Nedeianu S, Mambet C, Diaconu CC, Ruta SM. 2022. Kinetics and persistence of cellular and humoral immune responses to SARS-CoV-2 vaccine in healthcare workers with or without prior COVID-19. J Cell Mol Med 26:1293–1305. doi: 10.1111/jcmm.17186. - DOI - PMC - PubMed
-
- Gianfagna F, Veronesi G, Baj A, Dalla Gasperina D, Siclari S, Drago Ferrante F, Maggi F, Iacoviello L, Ferrario MM. 2022. Anti-SARS-CoV-2 antibody levels and kinetics of vaccine response: potential role for unresolved inflammation following recovery from SARS-CoV-2 infection. Sci Rep 12:385. doi: 10.1038/s41598-021-04344-y. - DOI - PMC - PubMed
-
- Pegu A, O'Connell SE, Schmidt SD, O'Dell S, Talana CA, Lai L, Albert J, Anderson E, Bennett H, Corbett KS, Flach B, Jackson L, Leav B, Ledgerwood JE, Luke CJ, Makowski M, Nason MC, Roberts PC, Roederer M, Rebolledo PA, Rostad CA, Rouphael NG, Shi W, Wang L, Widge AT, Yang ES, Beigel JH, Graham BS, Mascola JR, Suthar MS, McDermott AB, Doria-Rose NA, Arega J, Buchanan W, Elsafy M, Hoang B, Lampley R, Kolhekar A, Koo H, Luke C, Makhene M, Nayak S, Pikaart-Tautges R, Russell J, Sindall E, Kunwar P, Anderson EJ, Bechnak A, Bower M, Camacho-Gonzalez AF, mRNA-1273 Study Group , et al. 2021. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science 373:1372–1377. doi: 10.1126/science.abj4176. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

