GALNT2 promotes invasiveness of colorectal cancer cells partly through AXL
- PMID: 36409270
- PMCID: PMC9812829
- DOI: 10.1002/1878-0261.13347
GALNT2 promotes invasiveness of colorectal cancer cells partly through AXL
Abstract
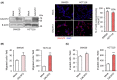
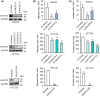
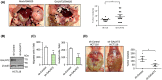
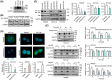
GalNAc-type O-glycosylation and its initiating GalNAc transferases (GALNTs) play crucial roles in a wide range of cellular behaviors. Among 20 GALNT members, GALNT2 is consistently associated with poor survival of patients with colorectal cancer in public databases. However, its clinicopathological significance in colorectal cancer remains unclear. In this study, immunohistochemistry showed that GALNT2 was overexpressed in colorectal tumors compared with the adjacent nontumor tissues. GALNT2 overexpression was associated with poor survival of colorectal cancer patients. Forced expression of GALNT2 promoted migration and invasion as well as peritoneal metastasis of colorectal cancer cells. In contrast, GALNT2 knockdown with siRNAs or knockout with CRISPR/Cas9 system suppressed these malignant properties. Interestingly, we found that GALNT2 modified O-glycans on AXL and determined AXL levels via the proteasome-dependent pathway. In addition, the GALNT2-promoted invasiveness was significantly reversed by AXL siRNAs. These findings suggest that GALNT2 promotes colorectal cancer invasion at least partly through AXL.
Keywords: AXL; GALNT; glycosylation; invasion.
© 2022 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Kawada K, Hasegawa S, Murakami T, Itatani Y, Hosogi H, Sonoshita M, et al. Molecular mechanisms of liver metastasis. Int J Clin Oncol. 2011;16:464–72. - PubMed
-
- Franko J, Shi Q, Meyers JP, Maughan TS, Adams RA, Seymour MT, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the analysis and research in cancers of the digestive system (ARCAD) database. Lancet Oncol. 2016;17:1709–19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

