Population-Level Impact of Omitting Axillary Lymph Node Dissection in Early Breast Cancer Women: Evidence from an Economic Evaluation in Germany
- PMID: 36409454
- PMCID: PMC9676848
- DOI: 10.1007/s40258-022-00771-8
Population-Level Impact of Omitting Axillary Lymph Node Dissection in Early Breast Cancer Women: Evidence from an Economic Evaluation in Germany
Abstract
Background: The American College of Surgeons Oncology Group Z0011 trial showed that complete axillary lymph node dissection (cALND) did not improve survival benefits in patients with one or two tumour-involved sentinel lymph nodes and undergoing breast conservation. Still, a considerable number of the Z0011-eligible patients continue to be treated with cALND in various countries. Given the potential economic gain from implementation of the Z0011 recommendations, we quantified population-level impacts of omitting cALND among Z0011-eligible patients in clinical practice.
Methods: This 2-year economic analysis adopted both the perspective of patients under statutory insurance and the societal perspective, using data collected prospectively from 179 German breast cancer units between 2008 and 2015. The estimation of cost savings and health gain relied on a single decision tree, which considered three scenarios: clinical practice at the baseline; actual implementation in routine care; and hypothetical full implementation in all eligible patients.
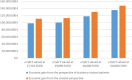
Results: Data for 188,909 patients with primary breast cancer were available, 13,741 (7.3%) of whom met the Z0011 inclusion criteria. The use of cALND decreased from 94.3% in 2010 to 46.9% in 2015, resulting in a gain of 335 quality-adjusted life-years and a saving of EUR50,334,756 for the society. Had cALND been omitted in all eligible patients, the total gain would have been more than double.
Conclusions: The implementation of the Z0011 recommendations resulted in substantial savings and health gain in Germany. Our findings suggest that it is beneficial to introduce additional policy measures to promote further uptake of the Z0011 recommendations in clinical practice.
© 2022. The Author(s).
Conflict of interest statement
There is no conflict of interest to declare.
Figures

References
-
- Giuliano AE, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, Saha S, Hunt KK, Morrow M, Ballman K. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252(3):426–433. doi: 10.1097/SLA.0b013e3181f08f32. - DOI - PMC - PubMed
-
- Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, McCall LM, Morrow M. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569–575. doi: 10.1001/jama.2011.90. - DOI - PMC - PubMed
-
- Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, Ollila DW, Hansen NM, Whitworth PW, Blumencranz PW, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (alliance) randomized clinical trial. JAMA. 2017;318(10):918–926. doi: 10.1001/jama.2017.11470. - DOI - PMC - PubMed
-
- Giuliano AE, Ballman K, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, Saha S, Morrow M, Hunt KK. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the American College of Surgeons Oncology Group (alliance) ACOSOG Z0011 randomized trial. Ann Surg. 2016;264(3):413–420. doi: 10.1097/SLA.0000000000001863. - DOI - PMC - PubMed
-
- Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P, Baratella P, Chifu C, Sargenti M, Intra M, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23–01): a phase 3 randomised controlled trial. Lancet Oncol. 2013;14(4):297–305. doi: 10.1016/S1470-2045(13)70035-4. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

