Model-based estimation of the impact on rotavirus disease of RV3-BB vaccine administered in a neonatal or infant schedule
- PMID: 36409459
- PMCID: PMC9746532
- DOI: 10.1080/21645515.2022.2139097
Model-based estimation of the impact on rotavirus disease of RV3-BB vaccine administered in a neonatal or infant schedule
Abstract
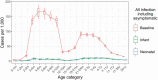
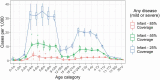
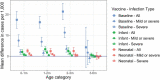
Rotavirus infection is a common cause of severe diarrheal disease and a major cause of deaths and hospitalizations among young children. Incidence of rotavirus has declined globally with increasing vaccine coverage. However, it remains a significant cause of morbidity and mortality in low-income countries where vaccine access is limited and efficacy is lower. The oral human neonatal vaccine RV3-BB can be safely administered earlier than other vaccines, and recent trials in Indonesia have demonstrated high efficacy. In this study, we use a stochastic individual-based model of rotavirus transmission and disease to estimate the anticipated population-level impact of RV3-BB following delivery according to either an infant (2, 4, 6 months) and neonatal (0, 2, 4 months) schedule. Using our model, which incorporated an age- and household-structured population and estimates of vaccine efficacy derived from trial data, we found both delivery schedules to be effective at reducing infection and disease. We estimated 95-96% reductions in infection and disease in children under 12 months of age when vaccine coverage is 85%. We also estimate high levels of indirect protection from vaccination, including 78% reductions in infection in adults over 17 years of age. Even for lower vaccine coverage of 55%, we estimate reductions of 84% in infection and disease in children under 12 months of age. While open questions remain about the drivers of observed lower efficacy in low-income settings, our model suggests RV3-BB could be effective at reducing infection and preventing disease in young infants at the population level.
Keywords: Indonesia; RV3-BB vaccine; Rotavirus; individual-based model; neonatal schedule.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
-
- Troeger C, Khalil IA, Rao PC, Cao S, Blacker BF, Ahmed T, Armah G, Bines JE, Brewer TG, Colombara DV, et al. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr. 2018;172(10):1–9. doi: 10.1001/jamapediatrics.2018.1960. - DOI - PMC - PubMed
-
- International Vaccine Access Center . Viewhub report: global vaccine introduction and implementation. Baltimore, MD: Johns Hopkins Bloomberg School of Public Health; 2021. https://view-hub.org/sites/default/files/2021-11/VIEW-hub_Report_Sept202...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
