Relative Validation of an Artificial Intelligence-Enhanced, Image-Assisted Mobile App for Dietary Assessment in Adults: Randomized Crossover Study
- PMID: 36409539
- PMCID: PMC9723975
- DOI: 10.2196/40449
Relative Validation of an Artificial Intelligence-Enhanced, Image-Assisted Mobile App for Dietary Assessment in Adults: Randomized Crossover Study
Abstract
Background: Thorough dietary assessment is essential to obtain accurate food and nutrient intake data yet challenging because of the limitations of current methods. Image-based methods may decrease energy underreporting and increase the validity of self-reported dietary intake. Keenoa is an image-assisted food diary that integrates artificial intelligence food recognition. We hypothesized that Keenoa is as valid for dietary assessment as the automated self-administered 24-hour recall (ASA24)-Canada and better appreciated by users.
Objective: We aimed to evaluate the relative validity of Keenoa against a 24-hour validated web-based food recall platform (ASA24) in both healthy individuals and those living with diabetes. Secondary objectives were to compare the proportion of under- and overreporters between tools and to assess the user's appreciation of the tools.
Methods: We used a randomized crossover design, and participants completed 4 days of Keenoa food tracking and 4 days of ASA24 food recalls. The System Usability Scale was used to assess perceived ease of use. Differences in reported intakes were analyzed using 2-tailed paired t tests or Wilcoxon signed-rank test and deattenuated correlations by Spearman coefficient. Agreement and bias were determined using the Bland-Altman test. Weighted Cohen κ was used for cross-classification analysis. Energy underreporting was defined as a ratio of reported energy intake to estimated resting energy expenditure <0.9.
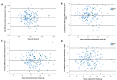
Results: A total of 136 participants were included (mean 46.1, SD 14.6 years; 49/136, 36% men; 31/136, 22.8% with diabetes). The average reported energy intakes (kcal/d) were 2171 (SD 553) in men with Keenoa and 2118 (SD 566) in men with ASA24 (P=.38) and, in women, 1804 (SD 404) with Keenoa and 1784 (SD 389) with ASA24 (P=.61). The overall mean difference (kcal/d) was -32 (95% CI -97 to 33), with limits of agreement of -789 to 725, indicating acceptable agreement between tools without bias. Mean reported macronutrient, calcium, potassium, and folate intakes did not significantly differ between tools. Reported fiber and iron intakes were higher, and sodium intake lower, with Keenoa than ASA24. Intakes in all macronutrients (r=0.48-0.73) and micronutrients analyzed (r=0.40-0.74) were correlated (all P<.05) between tools. Weighted Cohen κ scores ranged from 0.30 to 0.52 (all P<.001). The underreporting rate was 8.8% (12/136) with both tools. Mean System Usability Scale scores were higher for Keenoa than ASA24 (77/100, 77% vs 53/100, 53%; P<.001); 74.8% (101/135) of participants preferred Keenoa.
Conclusions: The Keenoa app showed moderate to strong relative validity against ASA24 for energy, macronutrient, and most micronutrient intakes analyzed in healthy adults and those with diabetes. Keenoa is a new, alternative tool that may facilitate the work of dietitians and nutrition researchers. The perceived ease of use may improve food-tracking adherence over longer periods.
Keywords: ASA24; Keenoa; automated self-administered 24-hour recall; dietary assessment; dietary intake; food diary; food records.
©Audrey Moyen, Aviva Ilysse Rappaport, Chloé Fleurent-Grégoire, Anne-Julie Tessier, Anne-Sophie Brazeau, Stéphanie Chevalier. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 21.11.2022.
Conflict of interest statement
Conflicts of Interest: AJT participated in the development of the Keenoa app and is the cofounder. The author did not receive any financial compensation for this study.
Figures
Similar articles
-
Validity and Usability of a Smartphone Image-Based Dietary Assessment App Compared to 3-Day Food Diaries in Assessing Dietary Intake Among Canadian Adults: Randomized Controlled Trial.JMIR Mhealth Uhealth. 2020 Sep 9;8(9):e16953. doi: 10.2196/16953. JMIR Mhealth Uhealth. 2020. PMID: 32902389 Free PMC article. Clinical Trial.
-
Performance and Feasibility of Recalls Completed Using the Automated Self-Administered 24-Hour Dietary Assessment Tool in Relation to Other Self-Report Tools and Biomarkers in the Interactive Diet and Activity Tracking in AARP (IDATA) Study.J Acad Nutr Diet. 2020 Nov;120(11):1805-1820. doi: 10.1016/j.jand.2020.06.015. Epub 2020 Aug 17. J Acad Nutr Diet. 2020. PMID: 32819883 Free PMC article.
-
Comparing the Usability of the Web-Based 24-h Dietary Recall R24W and ASA24-Canada-2018 among French-Speaking Adults from Québec.Nutrients. 2022 Oct 28;14(21):4543. doi: 10.3390/nu14214543. Nutrients. 2022. PMID: 36364803 Free PMC article. Clinical Trial.
-
Artificial Intelligence Applications to Measure Food and Nutrient Intakes: Scoping Review.J Med Internet Res. 2024 Nov 28;26:e54557. doi: 10.2196/54557. J Med Internet Res. 2024. PMID: 39608003 Free PMC article.
-
A review of the design and validation of web- and computer-based 24-h dietary recall tools.Nutr Res Rev. 2016 Dec;29(2):268-280. doi: 10.1017/S0954422416000172. Nutr Res Rev. 2016. PMID: 27955721 Review.
Cited by
-
Impact of glycemic variability on cognitive impairment, disordered eating behaviors and self-management skills in patients with type 1 diabetes: study protocol for a cross-sectional online study, the Sugar Swing study.BMC Endocr Disord. 2022 Nov 18;22(1):283. doi: 10.1186/s12902-022-01191-4. BMC Endocr Disord. 2022. PMID: 36401237 Free PMC article.
-
Mediterranean diet and time-restricted eating as a cardiac rehabilitation approach for patients with coronary heart disease and pre-diabetes: the DIABEPIC-1 protocol of a feasibility trial.BMJ Open. 2023 Oct 17;13(10):e073763. doi: 10.1136/bmjopen-2023-073763. BMJ Open. 2023. PMID: 37848307 Free PMC article.
-
Navigating next-gen nutrition care using artificial intelligence-assisted dietary assessment tools-a scoping review of potential applications.Front Nutr. 2025 Jan 23;12:1518466. doi: 10.3389/fnut.2025.1518466. eCollection 2025. Front Nutr. 2025. PMID: 39917741 Free PMC article. Review.
-
Surveying Nutrient Assessment with Photographs of Meals (SNAPMe): A Benchmark Dataset of Food Photos for Dietary Assessment.Nutrients. 2023 Nov 30;15(23):4972. doi: 10.3390/nu15234972. Nutrients. 2023. PMID: 38068830 Free PMC article.
-
Novel multimodal intervention for surgical prehabilitation on functional recovery and muscle characteristics in patients with non-small cell lung cancer: study protocol for a randomised controlled trial (MMP-LUNG).BMJ Open Respir Res. 2025 May 22;12(1):e002884. doi: 10.1136/bmjresp-2024-002884. BMJ Open Respir Res. 2025. PMID: 40404185 Free PMC article.
References
-
- Coulston A, Boushey C, Ferruzzi M. Nutrition in the Prevention and Treatment of Disease. 3rd edition. Cambridge, MA, USA: Elsevier Academic Press; 2012.
-
- Ortega RM, Pérez-Rodrigo C, López-Sobaler AM. Dietary assessment methods: dietary records. Nutr Hosp. 2015 Feb 26;31 Suppl 3:38–45. doi: 10.3305/nh.2015.31.sup3.8749. http://www.aulamedica.es/nh/pdf/8749.pdf - DOI - PubMed
-
- Willett W. Nutritional Epidemiology. 3rd edition. Oxford, UK: Oxford University Press; 2012.
-
- Gersovitz M, Madden JP, Smiciklas-Wright H. Validity of the 24-hr. dietary recall and seven-day record for group comparisons. J Am Diet Assoc. 1978 Jul;73(1):48–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources