Cerebrospinal fluid quinolinic acid is strongly associated with delirium and mortality in hip-fracture patients
- PMID: 36409557
- PMCID: PMC9843060
- DOI: 10.1172/JCI163472
Cerebrospinal fluid quinolinic acid is strongly associated with delirium and mortality in hip-fracture patients
Abstract
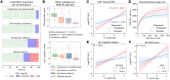
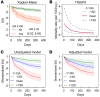
BACKGROUNDThe kynurenine pathway (KP) has been identified as a potential mediator linking acute illness to cognitive dysfunction by generating neuroactive metabolites in response to inflammation. Delirium (acute confusion) is a common complication of acute illness and is associated with increased risk of dementia and mortality. However, the molecular mechanisms underlying delirium, particularly in relation to the KP, remain elusive.METHODSWe undertook a multicenter observational study with 586 hospitalized patients (248 with delirium) and investigated associations between delirium and KP metabolites measured in cerebrospinal fluid (CSF) and serum by targeted metabolomics. We also explored associations between KP metabolites and markers of neuronal damage and 1-year mortality.RESULTSIn delirium, we found concentrations of the neurotoxic metabolite quinolinic acid in CSF (CSF-QA) (OR 2.26 [1.78, 2.87], P < 0.001) to be increased and also found increases in several other KP metabolites in serum and CSF. In addition, CSF-QA was associated with the neuronal damage marker neurofilament light chain (NfL) (β 0.43, P < 0.001) and was a strong predictor of 1-year mortality (HR 4.35 [2.93, 6.45] for CSF-QA ≥ 100 nmol/L, P < 0.001). The associations between CSF-QA and delirium, neuronal damage, and mortality remained highly significant following adjustment for confounders and multiple comparisons.CONCLUSIONOur data identified how systemic inflammation, neurotoxicity, and delirium are strongly linked via the KP and should inform future delirium prevention and treatment clinical trials that target enzymes of the KP.FUNDINGNorwegian Health Association and South-Eastern Norway Regional Health Authorities.
Keywords: Dementia; Inflammation; Metabolism; Neurological disorders; Psychiatric diseases.
Conflict of interest statement
Figures



Comment in
- The Kynurenine Pathway implicated in patient delirium: possible indications for indoleamine 2,3 dioxygenase inhibitors doi: 10.1172/JCI164577

