Anti-HK antibody inhibits the plasma contact system by blocking prekallikrein and factor XI activation in vivo
- PMID: 36409609
- PMCID: PMC10111358
- DOI: 10.1182/bloodadvances.2021006485
Anti-HK antibody inhibits the plasma contact system by blocking prekallikrein and factor XI activation in vivo
Abstract
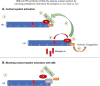
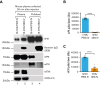
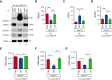
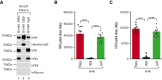
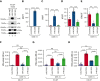
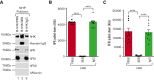
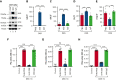
A dysregulated plasma contact system is involved in various pathological conditions, such as hereditary angioedema, Alzheimer disease, and sepsis. We previously showed that the 3E8 anti-high molecular weight kininogen (anti-HK) antibody blocks HK cleavage and bradykinin generation in human plasma ex vivo. Here, we show that 3E8 prevented not only HK cleavage but also factor XI (FXI) and prekallikrein (PK) activation by blocking their binding to HK in mouse plasma in vivo. 3E8 also inhibited contact system-induced bradykinin generation in vivo. Interestingly, FXII activation was also inhibited, likely because of the ability of 3E8 to block the positive feedback activation of FXII by kallikrein (PKa). In human plasma, 3E8 also blocked PK and FXI binding to HK and inhibited both thrombotic (FXI activation) and inflammatory pathways (PK activation and HK cleavage) of the plasma contact system activation ex vivo. Moreover, 3E8 blocked PKa binding to HK and dose-dependently inhibited PKa cleavage of HK. Our results reveal a novel strategy to inhibit contact system activation in vivo, which may provide an effective method to treat human diseases involving contact system dysregulation.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The 3E8 and 2B7 anti–HK antibodies have been licensed to MilliporeSigma.
Figures

References
-
- Schmaier AH. The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities. J Thromb Haemost. 2016;14(1):28–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

