Open-label pilot study of romiplostim for thrombocytopenia after autologous hematopoietic cell transplantation
- PMID: 36409612
- PMCID: PMC10130608
- DOI: 10.1182/bloodadvances.2022007838
Open-label pilot study of romiplostim for thrombocytopenia after autologous hematopoietic cell transplantation
Abstract
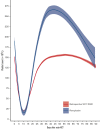
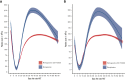
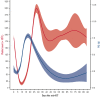
There are no standard treatments to prevent or hasten the recovery from severe conditioning-regimen-induced thrombocytopenia occurring after autologous hematopoietic cell transplantation (auto-HCT). We conducted an open-label, single-arm pilot study of romiplostim, a thrombopoietin receptor agonist, to enhance platelet recovery in patients with multiple myeloma or lymphoma undergoing auto-HCT. All patients were treated weekly with romiplostim starting day +1 after auto-HCT until the platelet count was >50 × 109/L without transfusion. Compared with contemporary retrospective data from romiplostim-naïve patients (N = 853), romiplostim-treated patients (N = 59) had a similar median number of days of grade 4 thrombocytopenia or days requiring transfusions, time to platelet engraftment, and number of platelets transfusions during the auto-HCT. However, romiplostim-treated patients had enhanced platelet recovery to normal values beginning at approximately day +15. In matched cohort multivariable analyses, romiplostim treatment was associated with higher platelet counts by an average of 40 × 109/L (95% confidence interval (CI) (14, 67), P = .003) and 118 × 109/L (95% CI [84, 152], P<.001) at days +21 and +30, respectively, compared with those of no romiplostim. Only 1 adverse event was deemed possibly attributable to romiplostim: a low-risk pulmonary embolism in a patient with multiple myeloma. In conclusion, romiplostim showed promising activity and safety after auto-HCT, but the improvement in platelet counts occurred later than the goal of shortening the duration and depth of the platelet nadir. This trial was registered at www.clinicaltrials.gov (#NCT04478123).
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: M.S. served as a paid consultant for McKinsey & Company, Angiocrine Bioscience, Inc., and Omeros Corporation; received research funding from Angiocrine Bioscience, Inc., and Omeros Corporation; served on ad hoc advisory boards for Kite, a Gilead Company; and received honoraria from i3Health and Medscape for CME-related activity. G.L.S. has received research funding to the institution from Janssen, Amgen, and Beyond Spring. C.S.S. has served as a paid consultant on advisory boards for Juno Therapeutics, Sanofi-Genzyme, Spectrum Pharmaceuticals, Novartis, Genmab, Precision Biosciences, Kite, a Gilead Company, Celgene/Bristol Myers Squibb (BMS), Gamida Cell, Karyopharm Therapeutics, and GlaxoSmithKline. He has received research funds for clinical trials from Juno Therapeutics, Celgene/BMS, BMS, Precision Biosciences, Actinium Pharmaceuticals, and Sanofi-Genzyme. D.J.C. receives research funding from Genentech. H.J.L. has served as a paid consultant for Takeda, Genzyme, Janssen, Karyopharm, Pfizer, Celgene, Caelum Biosciences, and has received research support from Takeda. O.B.L. served on advisory boards for MorphoSys, Inc. P.B.D. has served on an advisory board for Kite, a Gilead Company. M.-A.P. reports honoraria from AbbVie, Astellas, BMS, Celgene, Equilium, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Novartis, Nektar Therapeutics, Omeros, OrcaBio, Takeda, and VectivBio AG, Vor Biopharma; serves on the data and safety monitoring board for Cidara Therapeutics, Medigene, Sellas Life Sciences, and Servier, and the scientific advisory board of NexImmune; has ownership interests in NexImmune and Omeros; and received research support for clinical trials from Incyte, Kite/Gilead, Miltenyi Biotec, and Novartis. S.A.G. is a consultant and receives research funding from Amgen, Actinium, Celgene, Johnson & Johnson, and Takeda and is a consultant for Jazz Pharmaceuticals, Novartis, Kite, a Gilead Company, and Spectrum Pharmaceuticals. G.A.S. has served as a paid consultant for Anthos Therapeutics, Jiangsu Hengrui Pharmaceuticals Company Ltd., and Dova/Sobi Pharmaceuticals. The remaining authors declare no competing financial interests.
Figures
References
-
- Kanate AS, Majhail NS, Savani BN, et al. Indications for hematopoietic cell transplantation and immune effector cell therapy: guidelines from the american society for transplantation and cellular therapy. Biol Blood Marrow Transplant. 2020;26(7):1247–1256. - PubMed
-
- Wandt H, Schaefer-Eckart K, Wendelin K, et al. Therapeutic platelet transfusion versus routine prophylactic transfusion in patients with haematological malignancies: an open-label, multicentre, randomised study. Lancet. 2012;380(9850):1309–1316. - PubMed
-
- Stanworth SJ, Estcourt LJ, Powter G, et al. A no-prophylaxis platelet-transfusion strategy for hematologic cancers. N Engl J Med. 2013;368(19):1771–1780. - PubMed

