A systematic review of the biological mediators of fat taste and smell
- PMID: 36409650
- PMCID: PMC9678415
- DOI: 10.1152/physrev.00061.2021
A systematic review of the biological mediators of fat taste and smell
Abstract
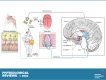
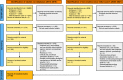
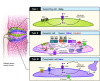
Taste and smell play a key role in our ability to perceive foods. Overconsumption of highly palatable energy-dense foods can lead to increased caloric intake and obesity. Thus there is growing interest in the study of the biological mediators of fat taste and associated olfaction as potential targets for pharmacologic and nutritional interventions in the context of obesity and health. The number of studies examining mechanisms underlying fat taste and smell has grown rapidly in the last 5 years. Therefore, the purpose of this systematic review is to summarize emerging evidence examining the biological mechanisms of fat taste and smell. A literature search was conducted of studies published in English between 2014 and 2021 in adult humans and animal models. Database searches were conducted using PubMed, EMBASE, Scopus, and Web of Science for key terms including fat/lipid, taste, and olfaction. Initially, 4,062 articles were identified through database searches, and a total of 84 relevant articles met inclusion and exclusion criteria and are included in this review. Existing literature suggests that there are several proteins integral to fat chemosensation, including cluster of differentiation 36 (CD36) and G protein-coupled receptor 120 (GPR120). This systematic review will discuss these proteins and the signal transduction pathways involved in fat detection. We also review neural circuits, key brain regions, ingestive cues, postingestive signals, and genetic polymorphism that play a role in fat perception and consumption. Finally, we discuss the role of fat taste and smell in the context of eating behavior and obesity.
Keywords: chemosensation; fat taste; obesity; oleogustus; olfaction.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures







References
-
- Dudine L, Canaletti C, Giudici F, Lunardelli A, Abram G, Santini I, Baroni V, Paris M, Pesavento V, Manganotti P, Ronchese F, Gregoretti B, Negro C. Investigation on the loss of taste and smell and consequent psychological effects: a cross-sectional study on healthcare workers who contracted the COVID-19 infection. Front Public Health 9: 666442, 2021. doi:10.3389/fpubh.2021.666442. - DOI - PMC - PubMed
-
- Hannum ME, Ramirez VA, Lipson SJ, Herriman RD, Toskala AK, Lin C, Joseph PV, Reed DR. Objective sensory testing methods reveal a higher prevalence of olfactory loss in COVID-19-positive patients compared to subjective methods: a systematic review and meta-analysis. Chem Senses 45: 865–874, 2020. doi:10.1093/chemse/bjaa064. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

