Multiplexed micronutrient, inflammation, and malarial antigenemia assessment using a plasma fractionation device
- PMID: 36409692
- PMCID: PMC9678258
- DOI: 10.1371/journal.pone.0277835
Multiplexed micronutrient, inflammation, and malarial antigenemia assessment using a plasma fractionation device
Abstract
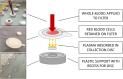
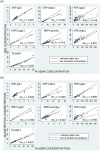
Processing and storing blood samples for future analysis of biomarkers can be challenging in resource limited environments. The preparation of dried blood spots (DBS) from finger-stick collection of whole blood is a widely used and established method as DBS are biosafe, and allow simpler field processing, storage, and transport protocols than serum or plasma. Therefore, DBS are commonly used in population surveys to assess infectious disease and/or micronutrient status. Recently, we reported that DBS can be used with the Q-plex™ Human Micronutrient 7-plex Array (MN 7-plex), a multiplexed immunoassay. This tool can simultaneously quantify seven protein biomarkers related to micronutrient deficiencies (iodine, iron and vitamin A), inflammation, and malarial antigenemia using plasma or serum. Serum ferritin, an iron biomarker, cannot be measured from DBS due to red blood cell (RBC) ferritin content confounding the results. In this study, we assess a simple blood fractionation tool that passively separates plasma from other blood components via diffusion through a membrane into a plasma collection disc (PCD). We evaluated the concordance of MN 7-plex analyte concentrations from matched panels of eighty-eight samples of PCD, DBS, and wet plasma prepared from anticoagulated venous whole blood. The results showed good correlations of >0.93 between the eluates from PCD and DBS for each analyte except ferritin; while correlations seen for plasma/PCD were weaker. However, the recovery rate of the analytes from the PCD were better than those from DBS. The serum ferritin measures from the PCD were highly correlated to wet plasma samples (0.85). This suggests that surveillance for iron status in low resource settings can be improved over the current methods restricted to only measuring sTfR in DBS. When used in combination with the MN 7-plex, all seven biomarkers can be simultaneously measured using eluates from the PCDs.
Copyright: © 2022 Brindle et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Measurement of micronutrient deficiency associated biomarkers in dried blood spots using a multiplexed immunoarray.PLoS One. 2019 Jan 8;14(1):e0210212. doi: 10.1371/journal.pone.0210212. eCollection 2019. PLoS One. 2019. PMID: 30620768 Free PMC article.
-
Simultaneous assessment of iodine, iron, vitamin A, malarial antigenemia, and inflammation status biomarkers via a multiplex immunoassay method on a population of pregnant women from Niger.PLoS One. 2017 Oct 5;12(10):e0185868. doi: 10.1371/journal.pone.0185868. eCollection 2017. PLoS One. 2017. PMID: 28982133 Free PMC article.
-
A multicenter analytical performance evaluation of a multiplexed immunoarray for the simultaneous measurement of biomarkers of micronutrient deficiency, inflammation and malarial antigenemia.PLoS One. 2021 Nov 4;16(11):e0259509. doi: 10.1371/journal.pone.0259509. eCollection 2021. PLoS One. 2021. PMID: 34735520 Free PMC article.
-
Pitfalls in the interpretation of blood tests used to assess and monitor micronutrient nutrition status.Nutr Clin Pract. 2023 Feb;38(1):56-69. doi: 10.1002/ncp.10924. Epub 2022 Nov 5. Nutr Clin Pract. 2023. PMID: 36335431 Review.
-
Inflammation and biomarkers of micronutrient status.Curr Opin Clin Nutr Metab Care. 2016 Nov;19(6):458-463. doi: 10.1097/MCO.0000000000000323. Curr Opin Clin Nutr Metab Care. 2016. PMID: 27583708 Review.
References
-
- World Health Organization. Worldwide prevalence of anaemia 1993–2005: WHO global database on anaemia. World Health Organization; 2008. https://apps.who.int/iris/handle/10665/43894.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

