Paeniclostridium sordellii uterine infection is dependent on the estrous cycle
- PMID: 36409774
- PMCID: PMC9721474
- DOI: 10.1371/journal.ppat.1010997
Paeniclostridium sordellii uterine infection is dependent on the estrous cycle
Abstract
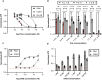
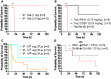
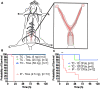
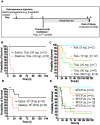
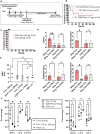
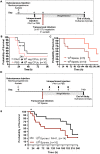
Human infections caused by the toxin-producing, anaerobic and spore-forming bacterium Paeniclostridium sordellii are associated with a treatment-refractory toxic shock syndrome (TSS). Reproductive-age women are at increased risk for P. sordellii infection (PSI) because this organism can cause intrauterine infection following childbirth, stillbirth, or abortion. PSI-induced TSS in this setting is nearly 100% fatal, and there are no effective treatments. TcsL, or lethal toxin, is the primary virulence factor in PSI and shares 70% sequence identity with Clostridioides difficile toxin B (TcdB). We therefore reasoned that a neutralizing monoclonal antibody (mAB) against TcdB might also provide protection against TcsL and PSI. We characterized two anti-TcdB mABs: PA41, which binds and prevents translocation of the TcdB glucosyltransferase domain into the cell, and CDB1, a biosimilar of bezlotoxumab, which prevents TcdB binding to a cell surface receptor. Both mABs could neutralize the cytotoxic activity of recombinant TcsL on Vero cells. To determine the efficacy of PA41 and CDB1 in vivo, we developed a transcervical inoculation method for modeling uterine PSI in mice. In the process, we discovered that the stage of the mouse reproductive cycle was a key variable in establishing symptoms of disease. By synchronizing the mice in diestrus with progesterone prior to transcervical inoculation with TcsL or vegetative P. sordellii, we observed highly reproducible intoxication and infection dynamics. PA41 showed efficacy in protecting against toxin in our transcervical in vivo model, but CDB1 did not. Furthermore, PA41 could provide protection following P. sordellii bacterial and spore infections, suggesting a path for further optimization and clinical translation in the effort to advance treatment options for PSI infection.
Copyright: © 2022 Bernard et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Genome-Wide CRISPR Screen Identifies Semaphorin 6A and 6B as Receptors for Paeniclostridium sordellii Toxin TcsL.Cell Host Microbe. 2020 May 13;27(5):782-792.e7. doi: 10.1016/j.chom.2020.03.007. Epub 2020 Apr 16. Cell Host Microbe. 2020. PMID: 32302524 Free PMC article.
-
Specific inhibition of phorbol ester-stimulated phospholipase D by Clostridium sordellii lethal toxin and Clostridium difficile toxin B-1470 in HEK-293 cells. Restoration by Ral GTPases.J Biol Chem. 1998 Mar 27;273(13):7413-22. doi: 10.1074/jbc.273.13.7413. J Biol Chem. 1998. PMID: 9516439
-
A Highly Specific Holin-Mediated Mechanism Facilitates the Secretion of Lethal Toxin TcsL in Paeniclostridiumsordellii.Toxins (Basel). 2022 Feb 8;14(2):124. doi: 10.3390/toxins14020124. Toxins (Basel). 2022. PMID: 35202151 Free PMC article.
-
Infections caused by Clostridium perfringens and Paeniclostridium sordellii after unsafe abortion.Lancet Infect Dis. 2023 Feb;23(2):e48-e55. doi: 10.1016/S1473-3099(22)00590-4. Epub 2022 Sep 22. Lancet Infect Dis. 2023. PMID: 36155670 Review.
-
Large Clostridial Toxins: Mechanisms and Roles in Disease.Microbiol Mol Biol Rev. 2021 Aug 18;85(3):e0006421. doi: 10.1128/MMBR.00064-21. Epub 2021 Jun 2. Microbiol Mol Biol Rev. 2021. PMID: 34076506 Free PMC article. Review.
Cited by
-
Zoonotic bacterial and parasitic intestinal pathogens in foxes, raccoons and other predators from eastern Germany.Environ Microbiol Rep. 2024 Jun;16(3):e13261. doi: 10.1111/1758-2229.13261. Environ Microbiol Rep. 2024. PMID: 38747071 Free PMC article.
-
Gut Microbial Adaptation to Varied Altitudes and Temperatures in Tibetan Plateau Yaks.Microorganisms. 2024 Jul 1;12(7):1350. doi: 10.3390/microorganisms12071350. Microorganisms. 2024. PMID: 39065118 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

