Inflammatory platelet production stimulated by tyrosyl-tRNA synthetase mimicking viral infection
- PMID: 36409883
- PMCID: PMC9860251
- DOI: 10.1073/pnas.2212659119
Inflammatory platelet production stimulated by tyrosyl-tRNA synthetase mimicking viral infection
Abstract
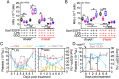
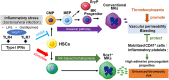
Platelets play a role not only in hemostasis and thrombosis, but also in inflammation and innate immunity. We previously reported that an activated form of tyrosyl-tRNA synthetase (YRSACT) has an extratranslational activity that enhances megakaryopoiesis and platelet production in mice. Here, we report that YRSACT mimics inflammatory stress inducing a unique megakaryocyte (MK) population with stem cell (Sca1) and myeloid (F4/80) markers through a mechanism dependent on Toll-like receptor (TLR) activation and type I interferon (IFN-I) signaling. This mimicry of inflammatory stress by YRSACT was studied in mice infected by lymphocytic choriomeningitis virus (LCMV). Using Sca1/EGFP transgenic mice, we demonstrated that IFN-I induced by YRSACT or LCMV infection suppressed normal hematopoiesis while activating an alternative pathway of thrombopoiesis. Platelets of inflammatory origin (Sca1/EGFP+) were a relevant proportion of those circulating during recovery from thrombocytopenia. Analysis of these "inflammatory" MKs and platelets suggested their origin in myeloid/MK-biased hematopoietic stem cells (HSCs) that bypassed the classical MK-erythroid progenitor (MEP) pathway to replenish platelets and promote recovery from thrombocytopenia. Notably, inflammatory platelets displayed enhanced agonist-induced activation and procoagulant activities. Moreover, myeloid/MK-biased progenitors and MKs were mobilized from the bone marrow, as evidenced by their presence in the lung microvasculature within fibrin-containing microthrombi. Our results define the function of YRSACT in platelet generation and contribute to elucidate platelet alterations in number and function during viral infection.
Keywords: inflammatory stress; megakaryocyte; platelet; tyrosyl-tRNA synthetase; viral infection.
Conflict of interest statement
Z.M.R. is Founder, President, and CEO, W.R. is Member of the Board, A.Z. is Chief Innovation Officer of MERU-VasImmune, Inc.; all have equity interest in the Company. S.K. and T.K. have equity interest in MERU-VasImmune, Inc., and J.N.O. is an employee of the company. X.-L.Y. and P.S. have financial interest in aTyr Pharma. aTyr works on therapeutic applications of tRNA synthetases and MERU VasImmune works on hematological tests. Neither aTyr Pharma nor MERU have any financial interest or intellectual property rights associated with the current work. The remaining authors declare no competing financial interests. Ellie Tzima, one of the reviewers, has financial interest in aTyr Pharma, but no interest specifically in this work.
Figures





References
-
- Ho-Tin-Noe B., Boulaftali Y., Camerer E., Platelets and vascular integrity: How platelets prevent bleeding in inflammation. Blood 131, 277–288 (2018). - PubMed
-
- Projahn D., Koenen R. R., Platelets: Key players in vascular inflammation. J. Leukoc. Biol. 92, 1167–1175 (2012). - PubMed
-
- Semple J. W., Italiano J. E. Jr., Freedman J., Platelets and the immune continuum. Nat. Rev. Immunol. 11, 264–274 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

