Temporal dynamics of the sensorimotor convergence underlying voluntary limb movement
- PMID: 36409890
- PMCID: PMC9860324
- DOI: 10.1073/pnas.2208353119
Temporal dynamics of the sensorimotor convergence underlying voluntary limb movement
Abstract
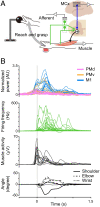
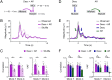
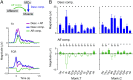
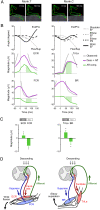
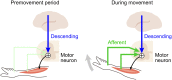
Descending motor drive and somatosensory feedback play important roles in modulating muscle activity. Numerous studies have characterized the organization of neuronal connectivity in which descending motor pathways and somatosensory afferents converge on spinal motor neurons as a final common pathway. However, how inputs from these two pathways are integrated into spinal motor neurons to generate muscle activity during actual motor behavior is unknown. Here, we simultaneously recorded activity in the motor cortices (MCx), somatosensory afferent neurons, and forelimb muscles in monkeys performing reaching and grasping movements. We constructed a linear model to explain the instantaneous muscle activity using the activity of MCx (descending input) and peripheral afferents (afferent input). Decomposition of the reconstructed muscle activity into each subcomponent indicated that muscle activity before movement onset could first be explained by descending input from mainly the primary motor cortex and muscle activity after movement onset by both descending and afferent inputs. Descending input had a facilitative effect on all muscles, whereas afferent input had a facilitative or suppressive effect on each muscle. Such antagonistic effects of afferent input can be explained by reciprocal effects of the spinal reflex. These results suggest that descending input contributes to the initiation of limb movement, and this initial movement subsequently affects muscle activity via the spinal reflex in conjunction with the continuous descending input. Thus, spinal motor neurons are subjected to temporally organized modulation by direct activation through the descending pathway and the lagged action of the spinal reflex during voluntary limb movement.
Keywords: decoding; motor cortex; muscle activity; spinal reflex; voluntary movement.
Conflict of interest statement
The authors declare no competing interest.
Figures



Similar articles
-
Future spinal reflex is embedded in primary motor cortex output.Sci Adv. 2024 Dec 20;10(51):eadq4194. doi: 10.1126/sciadv.adq4194. Epub 2024 Dec 18. Sci Adv. 2024. PMID: 39693430 Free PMC article.
-
[Reflex circuits of the spinal cord in man. Control during movement and their functional role (1)].Rev Neurol (Paris). 1984;140(11):605-14. Rev Neurol (Paris). 1984. PMID: 6239356 French.
-
Spinal functions in sensorimotor control of movements.Neurosurg Rev. 1990;13(3):179-85. doi: 10.1007/BF00313016. Neurosurg Rev. 1990. PMID: 2204840 Review.
-
Integration in descending motor pathways controlling the forelimb in the cat. 2. Convergence on neurones mediating disynaptic cortico-motoneuronal excitation.Exp Brain Res. 1976 Dec 22;26(5):521-40. doi: 10.1007/BF00238825. Exp Brain Res. 1976. PMID: 188674
-
Contributions to the understanding of gait control.Dan Med J. 2014 Apr;61(4):B4823. Dan Med J. 2014. PMID: 24814597 Review.
Cited by
-
Future spinal reflex is embedded in primary motor cortex output.Sci Adv. 2024 Dec 20;10(51):eadq4194. doi: 10.1126/sciadv.adq4194. Epub 2024 Dec 18. Sci Adv. 2024. PMID: 39693430 Free PMC article.
-
Autogenous cerebral processes: an invitation to look at the brain from inside out.Front Neural Circuits. 2023 Oct 19;17:1253609. doi: 10.3389/fncir.2023.1253609. eCollection 2023. Front Neural Circuits. 2023. PMID: 37941893 Free PMC article.
-
Anatomo-functional approach to multimodal motor mapping in diffuse glioma surgery: hierarchical networks.Brain Struct Funct. 2025 Jun 17;230(6):103. doi: 10.1007/s00429-025-02963-z. Brain Struct Funct. 2025. PMID: 40526141 Review.
-
Peripheral neural interfaces for reading high-frequency brain signals.Nat Biomed Eng. 2025 Jun 27. doi: 10.1038/s41551-025-01445-1. Online ahead of print. Nat Biomed Eng. 2025. PMID: 40579488 Review.
-
Volume conductor models for magnetospinography.Sci Rep. 2025 Jul 19;15(1):26258. doi: 10.1038/s41598-025-10770-z. Sci Rep. 2025. PMID: 40683938 Free PMC article.
References
-
- Nielsen J. B., Sensorimotor integration at spinal level as a basis for muscle coordination during voluntary movement in humans. J. Appl. Physiol. 96, 1961–1967 (2004). - PubMed
-
- McCrea D. A., Supraspinal and segmental interactions. Can. J. Physiol. Pharmacol. 74, 513–517 (1996). - PubMed
-
- Jankowska E., Interneuronal relay in spinal pathways from proprioceptors. Prog. Neurobiol. 38, 335–378 (1992). - PubMed
-
- Baldissera F., Hultborn H., Illert M., “Integration in Spinal Neuronal Systems” in Handbook of Physiology, The Nervous System, Motor Control, (American Physiological Society, 1977), pp. 509–595.
-
- Hultborn H., Spinal reflexes, mechanisms and concepts: From Eccles to Lundberg and beyond. Prog. Neurobiol. 78, 215–232 (2006). - PubMed

