Modulation of cGAS-STING signaling by PPARα in a mouse model of ischemia-induced retinopathy
- PMID: 36409895
- PMCID: PMC9860285
- DOI: 10.1073/pnas.2208934119
Modulation of cGAS-STING signaling by PPARα in a mouse model of ischemia-induced retinopathy
Abstract
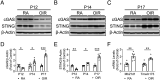
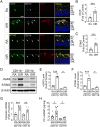
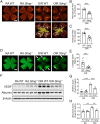
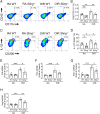
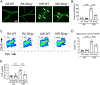
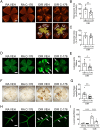
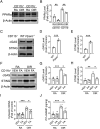
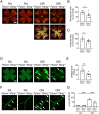
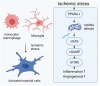
In ischemic retinopathy, overactivated retinal myeloid cells are a crucial driving force of pathological angiogenesis and inflammation. The cyclic GMP-AMP synthase (cGAS) and stimulator of interferon genes (STING) signaling are key regulators of inflammation. This study aims to investigate the association of cGAS-STING signaling with ischemic retinopathy and the regulation of its activation. We found that protein levels of cGAS and STING were markedly up-regulated in retinal myeloid cells isolated from mice with oxygen-induced retinopathy (OIR). Knockout of Sting and pharmacological inhibition of STING both alleviated retinal neovascularization (NV) and reduced retinal vascular leakage in OIR. Further, Sting knockout and STING inhibitor also alleviated leukocyte adhesion to retinal vasculature and infiltration into the retina as well as microglial activation in OIR. These results suggest that cGAS-STING signaling played a pathogenic role in retinal myeloid cell activation and NV in ischemic retinopathy. To identify the regulation of cGAS-STING signaling in OIR, we evaluated the role of transcription factor peroxisome proliferator-activated receptor α (PPARα). The results demonstrated that PPARα was down-regulated in OIR retinas, primarily in myeloid cells. Furthermore, Pparα knockout significantly up-regulated cGAS and STING levels in retinal CD11b+ cells, while PPARα agonist inhibited cGAS-STING signaling and cytosolic mitochondrial DNA (mtDNA) release, a causative feature for cGAS activation. Knockout of Sting ameliorated retinal NV, hyperpermeability, and leukostasis in Pparα-/- mice with OIR. These observations suggest that PPARα regulates cGAS-STING signaling, likely through mtDNA release, and thus, is a potential therapeutic target for ischemic retinopathy.
Keywords: PPARα; cGAS-STING signaling; ischemia-induced retinopathy; myeloid cells; neovascularization.
Conflict of interest statement
The authors declare no competing interest.
Figures
Similar articles
-
Regulation of Monocyte Activation by PPARα Through Interaction With the cGAS-STING Pathway.Diabetes. 2023 Jul 1;72(7):958-972. doi: 10.2337/db22-0654. Diabetes. 2023. PMID: 37058417 Free PMC article.
-
Therapeutic intervention in neuroinflammation for neovascular ocular diseases through targeting the cGAS-STING-necroptosis pathway.J Neuroinflammation. 2024 Jun 25;21(1):164. doi: 10.1186/s12974-024-03155-y. J Neuroinflammation. 2024. PMID: 38918759 Free PMC article.
-
Targeting mitochondrial DNA-STING-NF-κB Axis-mediated microglia activation by cryptotanshinone alleviates ischemic retinopathy.Phytomedicine. 2025 Jul;142:156779. doi: 10.1016/j.phymed.2025.156779. Epub 2025 Apr 18. Phytomedicine. 2025. PMID: 40279967
-
The cGAS-STING Pathway: Novel Perspectives in Liver Diseases.Front Immunol. 2021 Apr 29;12:682736. doi: 10.3389/fimmu.2021.682736. eCollection 2021. Front Immunol. 2021. PMID: 33995425 Free PMC article. Review.
-
cGAS-STING signaling in ischemic diseases.Clin Chim Acta. 2022 Jun 1;531:177-182. doi: 10.1016/j.cca.2022.04.003. Epub 2022 Apr 8. Clin Chim Acta. 2022. PMID: 35398249 Review.
Cited by
-
The cGAS-STING pathway: a therapeutic target in diabetes and its complications.Burns Trauma. 2024 Feb 2;12:tkad050. doi: 10.1093/burnst/tkad050. eCollection 2024. Burns Trauma. 2024. PMID: 38312740 Free PMC article. Review.
-
Design and Assessment of First-Generation Heterobifunctional PPARα/STING Modulators.ACS Med Chem Lett. 2024 Jul 22;15(8):1279-1286. doi: 10.1021/acsmedchemlett.4c00153. eCollection 2024 Aug 8. ACS Med Chem Lett. 2024. PMID: 39140058 Free PMC article.
-
Succinate-induced macrophage polarization and RBP4 secretion promote vascular sprouting in ocular neovascularization.J Neuroinflammation. 2023 Dec 21;20(1):308. doi: 10.1186/s12974-023-02998-1. J Neuroinflammation. 2023. PMID: 38129891 Free PMC article.
-
The BET PROTAC inhibitor dBET6 protects against retinal degeneration and inhibits the cGAS-STING in response to light damage.J Neuroinflammation. 2023 May 22;20(1):119. doi: 10.1186/s12974-023-02804-y. J Neuroinflammation. 2023. PMID: 37217935 Free PMC article.
-
The cGAS-STING pathway in diabetic retinopathy and age-related macular degeneration.Future Med Chem. 2023 Apr;15(8):717-729. doi: 10.4155/fmc-2022-0301. Epub 2023 May 11. Future Med Chem. 2023. PMID: 37166075 Free PMC article. Review.
References
-
- Ding J., Wong T. Y., Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr. Diab. Rep. 12, 346–354 (2012). - PubMed
-
- Wong W. L., et al. , Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob Health 2, e106–116 (2014). - PubMed
-
- Newman D. K., Surgical management of the late complications of proliferative diabetic retinopathy. Eye (Lond.) 24, 441–449 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

