Keratocytes migrate against flow with a roly-poly-like mechanism
- PMID: 36409912
- PMCID: PMC9889884
- DOI: 10.1073/pnas.2210379119
Keratocytes migrate against flow with a roly-poly-like mechanism
Abstract
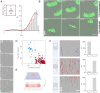
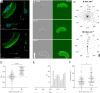
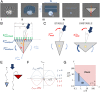
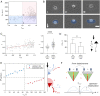
While cell migration can be directed by various mechanical cues such as force, deformation, stiffness, or flow, the associated mechanisms and functions may remain elusive. Single cell migration against flow, repeatedly reported with leukocytes, is arguably considered as active and mediated by integrin mechanotransduction, or passive and determined by a mechanical bias. Here, we reveal a phenotype of flow mechanotaxis with fish epithelial keratocytes that orient upstream or downstream at shear stresses around tens of dyn cm-2. We show that each cell has an intrinsic orientation that results from the mechanical interaction of flow with its morphology. The bulbous trailing edge of a keratocyte generates a hydrodynamical torque under flow that stabilizes an upstream orientation, just as the heavy lower edge of a roly-poly toy generates a gravitational torque that stabilizes an upright position. In turn, the wide and flat leading edge of keratocytes destabilizes upstream orientation, allowing the existence of two distinct phenotypes. To formalize these observations, we propose a simple mechanical model that considers keratocyte morphology as a hemisphere preceded by a wide thin sheet. Our findings show that this model can recapitulate the phase diagram of single cell orientation under flow without adjustable parameters. From a larger perspective, this passive mechanism of keratocytes flow mechanotaxis implies a potential absence of physiological function and evolution-driven process.
Keywords: directed migration; flow; keratocytes; mechanotaxis; mechanotransduction.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Carter S. B., Haptotaxis and the mechanism of cell motility. Nature 213, 256 (1967). - PubMed
-
- Dineur E., Note sur la sensibilité des leucocytes à l’électricité. Bull Séances Soc Belge Microsc. 18, 113–118 (1891).
-
- Blakemore R., Magnetotactic bacteria. Science 190, 377–379 (1975). - PubMed
-
- Sunyer R., Trepat X., Durotaxis. Curr. Biol. 30, R383–R387 (2020). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

