Activation of TREK-1 (K2P2.1) potassium channels protects against influenza A-induced lung injury
- PMID: 36410022
- PMCID: PMC9829483
- DOI: 10.1152/ajplung.00116.2022
Activation of TREK-1 (K2P2.1) potassium channels protects against influenza A-induced lung injury
Abstract
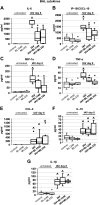
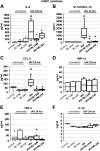
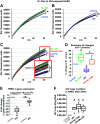
Influenza-A virus (IAV) infects yearly an estimated one billion people worldwide, resulting in 300,000-650,000 deaths. Preventive vaccination programs and antiviral medications represent the mainstay of therapy, but with unacceptably high morbidity and mortality rates, new targeted therapeutic approaches are urgently needed. Since inflammatory processes are commonly associated with measurable changes in the cell membrane potential (Em), we investigated whether Em hyperpolarization via TREK-1 (K2P2.1) K+ channel activation can protect against influenza-A virus (IAV)-induced pneumonia. We infected mice with IAV, which after 5 days caused 10-15% weight loss and a decrease in spontaneous activity, representing a clinically relevant infection. We then started a 3-day intratracheal treatment course with the novel TREK-1 activating compounds BL1249 or ML335. We confirmed TREK-1 activation with both compounds in untreated and IAV-infected primary human alveolar epithelial cells (HAECs) using high-throughput fluorescent imaging plate reader (FLIPR) assays. In mice, TREK-1 activation with BL1249 and ML335 counteracted IAV-induced histological lung injury and decrease in lung compliance and improved BAL fluid total protein levels, cell counts, and inflammatory IL-6, IP-10/CXCL-10, MIP-1α, and TNF-α levels. To determine whether these anti-inflammatory effects were mediated by activation of alveolar epithelial TREK-1 channels, we studied the effects of BL1249 and ML335 in IAV-infected HAEC, and found that TREK-1 activation decreased IAV-induced inflammatory IL-6, IP-10/CXCL10, and CCL-2 secretion. Dissection of TREK-1 downstream signaling pathways and construction of protein-protein interaction (PPI) networks revealed NF-κB1 and retinoic acid-inducible gene-1 (RIG-1) cascades as the most likely targets for TREK-1 protection. Therefore, TREK-1 activation may represent a novel therapeutic approach against IAV-induced lung injury.
Keywords: TREK-1 (KCNK2) ion channels; acute lung injury; cytokines; inflammation; influenza virus.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




Similar articles
-
K2P2.1 (TREK-1) potassium channel activation protects against hyperoxia-induced lung injury.Sci Rep. 2020 Dec 15;10(1):22011. doi: 10.1038/s41598-020-78886-y. Sci Rep. 2020. PMID: 33319831 Free PMC article.
-
BK Channels Regulate LPS-induced CCL-2 Release from Human Pulmonary Endothelial Cells.Am J Respir Cell Mol Biol. 2021 Feb;64(2):224-234. doi: 10.1165/rcmb.2020-0228OC. Am J Respir Cell Mol Biol. 2021. PMID: 33217242 Free PMC article.
-
Deficiency of the two-pore-domain potassium channel TREK-1 promotes hyperoxia-induced lung injury.Crit Care Med. 2014 Nov;42(11):e692-701. doi: 10.1097/CCM.0000000000000603. Crit Care Med. 2014. PMID: 25126877 Free PMC article.
-
Indoleamine 2,3-dioxygenase 1 drives epithelial cells ferroptosis in influenza-induced acute lung injury.Redox Biol. 2025 Apr;81:103572. doi: 10.1016/j.redox.2025.103572. Epub 2025 Feb 26. Redox Biol. 2025. PMID: 40023977 Free PMC article. Review.
-
The pathogenesis of influenza in intact alveoli: virion endocytosis and its effects on the lung's air-blood barrier.Front Immunol. 2024 Jan 26;15:1328453. doi: 10.3389/fimmu.2024.1328453. eCollection 2024. Front Immunol. 2024. PMID: 38343548 Free PMC article. Review.
Cited by
-
Two-Pore-Domain Potassium Channel TREK-1 Mediates Pulmonary Fibrosis through Macrophage M2 Polarization and by Direct Promotion of Fibroblast Differentiation.Biomedicines. 2023 Apr 26;11(5):1279. doi: 10.3390/biomedicines11051279. Biomedicines. 2023. PMID: 37238950 Free PMC article.
-
Mechanisms of impaired alveolar fluid clearance.Anat Rec (Hoboken). 2025 Apr;308(4):1026-1039. doi: 10.1002/ar.25166. Epub 2023 Jan 23. Anat Rec (Hoboken). 2025. PMID: 36688689 Free PMC article. Review.
-
Pro-Reparative Effects of KvLQT1 Potassium Channel Activation in a Mouse Model of Acute Lung Injury Induced by Bleomycin.Int J Mol Sci. 2025 Aug 7;26(15):7632. doi: 10.3390/ijms26157632. Int J Mol Sci. 2025. PMID: 40806760 Free PMC article.
-
Pharmacological activation of BK channels protects against LPS-induced pneumonia.Sci Rep. 2025 Aug 19;15(1):30307. doi: 10.1038/s41598-025-08902-6. Sci Rep. 2025. PMID: 40830381 Free PMC article.
-
Two-pore potassium channel TREK-1 (K2P2.1) regulates NLRP3 inflammasome activity in macrophages.Am J Physiol Lung Cell Mol Physiol. 2024 Mar 1;326(3):L367-L376. doi: 10.1152/ajplung.00313.2023. Epub 2024 Jan 22. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 38252657 Free PMC article.
References
-
- Lobo SM, Watanabe ASA, Salomão MLM, Queiroz F, Gandolfi JV, de Oliveira NE, Covello LHS, Sacillotto GH, de Godoy LG, Simões ES, Frini ICM, Da Silva Teixeira RER, Furlan NP, Dutra KR, Nogueira ML. Excess mortality is associated with influenza A (H1N1) in patients with severe acute respiratory illness. J Clin Virol 116: 62–68, 2019. doi:10.1016/j.jcv.2019.05.003. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

