Flammulina velutipes stem regulates oxidative damage and synthesis of yolk precursors in aging laying hens by regulating the liver-blood-ovary axis
- PMID: 36410067
- PMCID: PMC9678783
- DOI: 10.1016/j.psj.2022.102261
Flammulina velutipes stem regulates oxidative damage and synthesis of yolk precursors in aging laying hens by regulating the liver-blood-ovary axis
Abstract
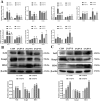
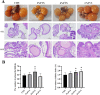
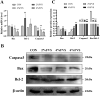
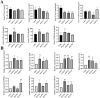
Egg production levels in late laying hens are negatively correlated with increasing age. Decreased liver and ovarian function in aging laying hens is accompanied by decreased antioxidant capacity, reproductive hormone levels, and follicular development, resulting in decreased synthesis of yolk precursors. The golden needle mushroom (Flammulina velutipes) has been reported to exhibit anti-inflammatory, antioxidant, and hypolipidemic properties. We aimed to reveal the therapeutic effects of F. velutipes stem (FVS) on liver-blood-ovary axis and investigate the underlying mechanisms. A total of 360 sixty-seven-wk-old laying hens were randomized into 4 treatment groups: 1) basal maize-soybean meal diet (CON); 2) basal maize + 20 g/kg FVS (2% FVS); 3) basal maize + 40 g/kg FVS (4% FVS); and 4) basal maize + 60 g/kg FVS (6% FVS). FVS groups demonstrated significantly increased egg production and ovarian development compared with the CON group. The addition of FVS increased the levels of antioxidant enzymes (GSH-Px, T-SOD, and T-AOC) in the liver, serum, and ovaries and decreased malondialdehyde levels by regulating the expression of proteins related to the Keap1-Nrf2/ARE signaling pathway. Additionally, FVS significantly decreased ovarian apoptosis by regulating Bax, Bcl-2, and caspase3 mRNA and protein expression levels. FVS significantly increased the expression levels of estradiol, progesterone, luteinizing hormone, and follicle stimulating hormone and their respective receptors. With increased levels of estradiol transported to the liver through the bloodstream, targeted binding to estrogen receptor (ER)-α and ER-β led to significant increases in ApoVLDL II, ApoB, and VTG II mRNA expression associated with yolk precursor synthesis. FVS decreased the levels of triglyceride and total cholesterol and significantly increased the expression of lipid metabolism, and transport-related mRNAs (FAS, PPAR-a/γ, and MTTP) in the liver. Therefore, the dietary supplementation of FVS can maintain the productive performance of aging laying hens by alleviating the degree of oxidative stress and regulating the transport of functional substances along the liver-blood-ovary axis, thereby improving the synthesis of yolk precursors.
Keywords: Flammulina velutipes stem; liver–blood–ovary signal axis; oxidative stress; reproductive hormone; yolk precursor synthesis.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Dietary Puerariae Lobatae Radix polysaccharides improve ovarian function and reproductive efficiency in laying hens with fatty liver hemorrhagic syndrome.Poult Sci. 2025 May;104(5):105062. doi: 10.1016/j.psj.2025.105062. Epub 2025 Mar 18. Poult Sci. 2025. PMID: 40120252 Free PMC article.
-
Polysaccharide from Hericium erinaceus improved laying performance of aged hens by promoting yolk precursor synthesis and follicle development via liver-blood-ovary axis.Poult Sci. 2024 Jul;103(7):103810. doi: 10.1016/j.psj.2024.103810. Epub 2024 Apr 30. Poult Sci. 2024. PMID: 38749108 Free PMC article.
-
Dietary Se-enrich Cardamine violifolia supplementation decreases lipid deposition and improves antioxidant status in the liver of aging laying hens.Poult Sci. 2025 Jan;104(1):104620. doi: 10.1016/j.psj.2024.104620. Epub 2024 Dec 2. Poult Sci. 2025. PMID: 39647356 Free PMC article.
-
Role of the Nrf2 Signaling Pathway in Ovarian Aging: Potential Mechanism and Protective Strategies.Int J Mol Sci. 2023 Aug 28;24(17):13327. doi: 10.3390/ijms241713327. Int J Mol Sci. 2023. PMID: 37686132 Free PMC article. Review.
-
Interacting Networks of the Hypothalamic-Pituitary-Ovarian Axis Regulate Layer Hens Performance.Genes (Basel). 2023 Jan 4;14(1):141. doi: 10.3390/genes14010141. Genes (Basel). 2023. PMID: 36672882 Free PMC article. Review.
Cited by
-
Effects of herbal dregs supplementation of Salvia miltiorrhiza and Isatidis Radix residues improved production performance and gut microbiota abundance in late-phase laying hens.Front Vet Sci. 2024 May 3;11:1381226. doi: 10.3389/fvets.2024.1381226. eCollection 2024. Front Vet Sci. 2024. PMID: 38764854 Free PMC article.
-
Effects of the Salvia miltiorrhiza, Ligustrum lucidum, and Taraxacum mongolicum ultra-fine powder formula on meat quality of aged layers by multi-omics.Poult Sci. 2025 Feb;104(2):104783. doi: 10.1016/j.psj.2025.104783. Epub 2025 Jan 5. Poult Sci. 2025. PMID: 39823836 Free PMC article.
-
Dietary Puerariae Lobatae Radix polysaccharides improve ovarian function and reproductive efficiency in laying hens with fatty liver hemorrhagic syndrome.Poult Sci. 2025 May;104(5):105062. doi: 10.1016/j.psj.2025.105062. Epub 2025 Mar 18. Poult Sci. 2025. PMID: 40120252 Free PMC article.
-
Multi-Omics Reveals Molecular and Genetic Mechanisms Underlying Egg Albumen Quality Decline in Aging Laying Hens.Int J Mol Sci. 2025 Aug 15;26(16):7876. doi: 10.3390/ijms26167876. Int J Mol Sci. 2025. PMID: 40869199 Free PMC article.
-
Graded levels of Eimeria infection modulated gut physiology and temporarily ceased the egg production of laying hens at peak production.Poult Sci. 2024 Jan;103(1):103229. doi: 10.1016/j.psj.2023.103229. Epub 2023 Nov 2. Poult Sci. 2024. PMID: 38007903 Free PMC article.
References
-
- Amevor F.K., Cui Z., Du X., Ning Z., Shu G., Jin N., Deng X., Tian Y., Zhang Z., Kang X., Xu D., You G., Zhang Y., Li D., Wang Y., Zhu Q., Zhao X. Combination of quercetin and vitamin e supplementation promotes yolk precursor synthesis and follicle development in aging breeder hens via liver-blood-ovary signal axis. Animals (Basel) 2021;11:1915. - PMC - PubMed
-
- Barber D.L., Sanders E.J., Aebersold R., Schneider W.J. The receptor for yolk lipoprotein deposition in the chicken oocyte. J. Biol. Chem. 1991;266:18761–18770. - PubMed
-
- Chen M., Mahfuz S., Cui Y., Jia L., Liu Z., Song H. The antioxidant status of serum and egg yolk in layer fed with mushroom stembase (Flammulina velutipes) Pak. J. Zool. 2019;52:389–392.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

