Magnesium in chronic haemodialysis (MAGIC-HD): a study protocol for a randomised controlled trial to determine feasibility and safety of using increased dialysate magnesium concentrations to increase plasma magnesium concentrations in people treated with haemodialysis
- PMID: 36410806
- PMCID: PMC9680141
- DOI: 10.1136/bmjopen-2022-063524
Magnesium in chronic haemodialysis (MAGIC-HD): a study protocol for a randomised controlled trial to determine feasibility and safety of using increased dialysate magnesium concentrations to increase plasma magnesium concentrations in people treated with haemodialysis
Abstract
Introduction: People treated with haemodialysis are at increased risk for all-cause and cardiovascular mortality. Plasma magnesium concentration has been inversely associated with these risks. Therefore, plasma magnesium may be a new modifiable risk factor and an increase of dialysate magnesium concentration may be an easy, safe and effective way to increase plasma magnesium concentrations. Detailed information on modulating dialysate magnesium concentrations is limited in literature. Primary objective of this study is to determine the safety and feasibility to increase plasma magnesium concentrations in people treated with haemodialysis by means of sequentially increasing concentration of magnesium in the dialysate.
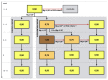
Methods and analysis: In this randomised double-blinded standard of care controlled trial, 53 persons treated with haemodialysis will be randomly allocated 2:1 to either a stepwise individually titrated increase of dialysate magnesium concentration from 0.50 to 0.75 to 1.00 mmol/L during 8 weeks, or a standard dialysate magnesium concentration of 0.50 mmol/L. Other study measurements include dietary records, questionnaires, ECG, Holter registration and pulse wave velocity. The primary endpoint is predialysis plasma magnesium after the long interdialytic interval at the end of week 8. In addition, the predictive effect of dialysate magnesium concentration and other baseline parameters and dialysis characteristics on plasma magnesium concentration will be explored using linear mixed models. Safety endpoint is defined by the occurrence of hypermagnesemia above 1.25 mmol/L, or bradycardia or prolonged QTc interval detected on the ECG.
Ethics and dissemination: The study is conducted in accordance with the declaration of Helsinki as revised in 2013 and was approved by the Ethical Committee of the VU University Medical Centre. The results of the study will be disseminated by publication in peer-reviewed scientific journals and presentation at national or international conferences in the field of interest.
Trial registration number: NTR6568/NL6393.
Keywords: dialysis; end stage renal failure; nephrology.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MGV received research grants from Vifor, Amgen, Fresenius, and acted as consultant for Medice, Astra Zeneca, Vifor, Amgen, Fresenius, Otsuma, Kyowa Kirin.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
