Sustained Low Relapse Rate With Highly Variable B-Cell Repopulation Dynamics With Extended Rituximab Dosing Intervals in Multiple Sclerosis
- PMID: 36411076
- PMCID: PMC9749930
- DOI: 10.1212/NXI.0000000000200056
Sustained Low Relapse Rate With Highly Variable B-Cell Repopulation Dynamics With Extended Rituximab Dosing Intervals in Multiple Sclerosis
Erratum in
-
Sustained Low Relapse Rate With Highly Variable B-Cell Repopulation Dynamics With Extended Rituximab Dosing Intervals in Multiple Sclerosis.Neurol Neuroimmunol Neuroinflamm. 2024 May;11(3):e200252. doi: 10.1212/NXI.0000000000200252. Epub 2024 Apr 5. Neurol Neuroimmunol Neuroinflamm. 2024. PMID: 38579190 Free PMC article. No abstract available.
Abstract
Background and objectives: B cell-depleting therapies are highly effective in relapsing-remitting multiple sclerosis (RRMS) but are associated with increased infection risk and blunted humoral vaccination responses. Extension of dosing intervals may mitigate such negative effects, but its consequences on MS disease activity are yet to be ascertained. The objective of this study was to determine clinical and neuroradiologic disease activity, as well as B-cell repopulation dynamics, after implementation of extended rituximab dosing in RRMS.
Methods: We conducted a prospective observational study in a specialized-care, single-center setting, including patients with RRMS participating in the COMBAT-MS and MultipleMS observational drug trials, who had received at least 2 courses of rituximab (median follow-up 4.2 years, range 0.1-8.9 years). Using Cox regression, hazard ratios (HRs) of clinical relapse and/or contrast-enhancing lesions on MRI were calculated in relation to time since last dose of rituximab.
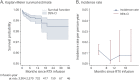
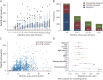
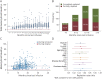
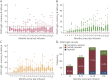
Results: A total of 3,904 dose intervals were accumulated in 718 patients and stratified into 4 intervals: <8, ≥8 to 12, ≥12 to 18, and ≥18 months. We identified 24 relapses of which 20 occurred within 8 months since previous infusion and 4 with intervals over 8 months. HRs for relapse when comparing ≥8 to 12, ≥12 to 18, and ≥18 months with <8 months since last dose were 0.28 (95% CI 0.04-2.10), 0.38 (95% CI 0.05-2.94), and 0.89 (95% CI 0.20-4.04), respectively, and thus nonsignificant. Neuroradiologic outcomes mirrored relapse rates. Dynamics of total B-cell reconstitution varied considerably, but median total B-cell counts reached lower level of normal after 12 months and median memory B-cell counts after 16 months.
Discussion: In this prospective cohort of rituximab-treated patients with RRMS exposed to extended dosing intervals, we could not detect a relation between clinical or neuroradiologic disease activity and time since last infusion. Total B- and memory B-cell repopulation kinetics varied considerably. These findings, relevant for assessing risk-mitigation strategies with anti-CD20 therapies in RRMS, suggest that relapse risk remains low with extended infusion intervals. Further studies are needed to investigate the relation between B-cell repopulation dynamics and adverse event risks associated with B-cell depletion.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
