Bacterial chemotaxis in human diseases
- PMID: 36411201
- PMCID: PMC11238666
- DOI: 10.1016/j.tim.2022.10.007
Bacterial chemotaxis in human diseases
Abstract
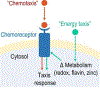
To infect and cause disease, bacterial pathogens must localize to specific regions of the host where they possess the metabolic and defensive acumen for survival. Motile flagellated pathogens exercise control over their localization through chemotaxis to direct motility based on the landscape of exogenous nutrients, toxins, and molecular cues sensed within the host. Here, we review advances in understanding the roles chemotaxis plays in human diseases. Chemotaxis drives pathogen colonization to sites of inflammation and injury and mediates fitness advantages through accessing host-derived nutrients from damaged tissue. Injury tropism may worsen clinical outcomes through instigating chronic inflammation and subsequent cancer development. Inhibiting bacterial chemotactic systems could act synergistically with antibacterial medicines for more effective and specific eradication.
Keywords: bacterial pathogenesis; chemotaxis; chronic inflammation; motility.
Copyright © 2022. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of interests A.B. owns Amethyst Antimicrobials, LLC. Funding for this work was provided by NIAID under award number 1K99AI148587 (A.B.).
Figures





References
-
- Erhardt M, “Strategies to Block Bacterial Pathogenesis by Interference with Motility and Chemotaxis,” in How to Overcome the Antibiotic Crisis : Facts, Challenges, Technologies and Future Perspectives, Stadler M and Dersch P, Eds. Cham: Springer International Publishing, 2016, pp. 185–205. doi: 10.1007/82_2016_493. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

