Developing a new sepsis screening tool based on lymphocyte count, international normalized ratio and procalcitonin (LIP score)
- PMID: 36411279
- PMCID: PMC9678875
- DOI: 10.1038/s41598-022-16744-9
Developing a new sepsis screening tool based on lymphocyte count, international normalized ratio and procalcitonin (LIP score)
Abstract
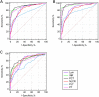
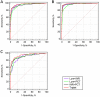
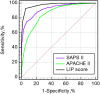
Exploring an effective sepsis screening tool that can be widely implemented is important for improving the prognosis of sepsis worldwide. This study aimed to develop a new simple screening tool for sepsis (LIP scoring system) that includes the peripheral blood lymphocyte count, international normalized ratio, and procalcitonin level. In a single-center, prospective, observational study, 444 acute sepsis inpatients and 444 nonsepsis inpatients were ultimately included based on the Sepsis-3 and exclusion criteria. The differences in the Lym, INR, PCT level and other clinical biomarkers were compared between the two groups. Univariable and multivariable logistic regression analyses and receiver operating characteristic analysis were used to establish a LIP screening tool for sepsis with a combination of biomarkers. The Kappa and McNemar tests were used to evaluate the differences between the LIP screening results (LIP score ≥ 3) and Sepsis-3 criteria (SOFA score ≥ 2). Logistic regression analysis showed that the lymphocyte count, INR, PCT level, platelets, neutrophil/lymphocyte ratio (NLR) and prothrombin time (PT) were independent risk factors for the development of sepsis. The ROC analysis showed that the lymphocyte count, INR, and PCT level had high area under the ROC curve values (AUROC (95% CI): Lym 0.84 (0.810-0.860), INR 0.921 (0.902-0.938), PCT level 0.928 (0.909-0.944)). The LIP tool had satisfactory screening efficacy for sepsis (sensitivity, 92.8%; specificity, 94.1%), and a LIP score equal to or greater than 3 points had good agreement with Sepsis-3 criteria in the diagnosis of sepsis (Kappa = 0862 in the Kappa test and P = 0.512 in the McNemar test). The LIP tool has satisfactory sensitivity and specificity for sepsis screening, and it can be used for rapid screening of patients with sepsis in outpatient and emergency departments or in economically underdeveloped areas with limited resources.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am. J. Respir. Crit. Care Med. 2016;193(3):259–272. doi: 10.1164/rccm.201504-0781OC. - DOI - PubMed
-
- Machado FR, Cavalcanti AB, Monteiro MB, Sousa JL, Bossa A, Bafi AT, Dal-Pizzol F, Freitas FGR, Lisboa T, Westphal GA, et al. Predictive accuracy of the quick sepsis-related organ failure assessment score in Brazil. A prospective multicenter study. Am. J. Respir. Crit. Care Med. 2020;201(7):789–798. doi: 10.1164/rccm.201905-0917OC. - DOI - PMC - PubMed
-
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American college of chest physicians/society of critical care medicine. Chest. 1992;101(6):1644–1655. doi: 10.1378/chest.101.6.1644. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2021MSXM033/Chongqing Medical Scientific Research Project (Joint Project of Chongqing Health Commission and Science and Technology Bureau)
- 2019MSXM019/Chongqing Medical Scientific Research Project (Joint Project of Chongqing Health Commission and Science and Technology Bureau)
- cstc2020jcyj-msxmX0124/Chongqing Natural Science Foundation Project
- X1-2611/COVID-19 Emergency Projects of Chongqing Medical University
LinkOut - more resources
Full Text Sources
Medical

