Radiotherapy in combination with CD47 blockade elicits a macrophage-mediated abscopal effect
- PMID: 36411318
- PMCID: PMC9701141
- DOI: 10.1038/s43018-022-00456-0
Radiotherapy in combination with CD47 blockade elicits a macrophage-mediated abscopal effect
Abstract
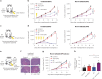
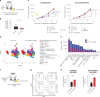
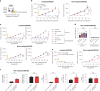
Radiation therapy is a mainstay of cancer treatment but does not always lead to complete tumor regression. Here we combine radiotherapy with blockade of the 'don't-eat-me' cell-surface molecule CD47 in small cell lung cancer (SCLC), a highly metastatic form of lung cancer. CD47 blockade potently enhances the local antitumor effects of radiotherapy in preclinical models of SCLC. Notably, CD47 blockade also stimulates off-target 'abscopal' effects inhibiting non-irradiated SCLC tumors in mice receiving radiation. These abscopal effects are independent of T cells but require macrophages that migrate into non-irradiated tumor sites in response to inflammatory signals produced by radiation and are locally activated by CD47 blockade to phagocytose cancer cells. Similar abscopal antitumor effects were observed in other cancer models treated with radiation and CD47 blockade. The systemic activation of antitumor macrophages following radiotherapy and CD47 blockade may be particularly important in patients with cancer who suffer from metastatic disease.
© 2022. The Author(s).
Conflict of interest statement
J.S. and I.L.W. licensed a patent to Forty Seven/Gilead on the use of CD47-blocking strategies in SCLC. J.S. has equity in and is an advisor for, DISCO Pharmaceuticals. I.L.W. is a director, stockholder and consultant in Forty Seven, a public company that was involved in CD47-based immunotherapy of cancer but was acquired by Gilead. At the time of this submission, I.L.W. has no formal relationship with Gilead. I.L.W. is a co-founder, Director and consultant for Bitterroot Bio, a company working on atherosclerosis from the anti-CD47 angle. I.L.W. is also a co-founder, Director and consultant for PHeast, a company testing new ‘don’t eat me’ signals with more limited cancer type distributions, including CD24. I.L.W. is co-founding 48, a company establishing antibody cocktails to prepare blood-forming stem cell recipients, both autologous and allogeneic, for hematopoietic stem cell engraftment without any radiation or chemotherapy. None of these companies fund the Weissman laboratory. I.L.W. is also on the scientific advisory board of Appia, a CAR-iNK company for cancer therapies. Y.N., E.E.G. and J.S. filed a patent based on these data (PCT/US2021/046548). A.T. reports research funding to the institution from the following entities: EMD Serono Research & Development Institute; AstraZeneca; Tarveda Therapeutics; Immunomedics and Prolynx. The authors declare no other competing interests.
Figures














References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

