Anti-Helicobacter pylori activity of potential probiotic Lactiplantibacillus pentosus SLC13
- PMID: 36411410
- PMCID: PMC9677914
- DOI: 10.1186/s12866-022-02701-z
Anti-Helicobacter pylori activity of potential probiotic Lactiplantibacillus pentosus SLC13
Abstract
Background: Here, we aimed to evaluate and compare the anti-Helicobacter pylori activity of potential probiotic Lactiplantibacillus pentosus SLC13 to Lactobacillus gasseri BCRC 14619 T and Lacticaseibacillus rhamnosus LGG. Phenotypic assays including growth curve, cell adhesion, and cellular cytotoxicity were performed to characterize SLC13. Anti-H. pylori activity of lactobacilli was determined by the disk diffusion method and co-culture assay. Exopolysaccharide (EPS) was extracted from lactobacilli to test its immune modulation activity, and IL-8 expression in AGS and GES-1 was determined by RT-qPCR.
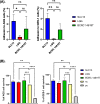
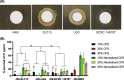
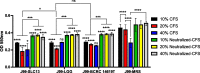
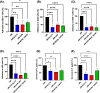
Results: All three lactobacilli strains were tolerant to the simulated gastrointestinal conditions. SLC13 showed the highest adhesion ability to AGS and GES-1 cells, compared to LGG and BCRC 14619 T. The coculture assays of SLC13, LGG, and BCRC 14619 T with cells for 4 h showed no significant cytotoxic effects on cells. All tested strains exhibited an inhibitory effect against H. pylori J99. The cell-free supernatant (CFS) of three strains showed activity to inhibit H. pylori urease activity in a dose-dependent manner and the CFS of SLC13 had the highest urease inhibitory activity, compared to LGG and BCRC 14619 T. Only the treatment of AGS cells with SLC13 EPS significantly decreased the IL-8 expression induced by H. pylori infection as compared to cells treated with LGG and BCRC 14619 T EPS.
Conclusions: SLC13 possesses potent antimicrobial activity against H. pylori growth, infection, and H. pylori-induced inflammation. These results suggest that SLC13 and its derivatives have the potential as alternative agents against H. pylori infection and alleviate inflammatory response.
Keywords: Adhesion; Exopolysaccharide; Helicobacter pylori; Lactiplantibacillus pentosus; Probiotic; Urease.
© 2022. The Author(s).
Conflict of interest statement
All authors have no conflicts of interest to declare.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

