OTUD4-mediated GSDME deubiquitination enhances radiosensitivity in nasopharyngeal carcinoma by inducing pyroptosis
- PMID: 36411454
- PMCID: PMC9677691
- DOI: 10.1186/s13046-022-02533-9
OTUD4-mediated GSDME deubiquitination enhances radiosensitivity in nasopharyngeal carcinoma by inducing pyroptosis
Abstract
Background: Radioresistance is the primary cause of nasopharyngeal carcinoma (NPC) treatment failure. Previous studies have focused on the deficits in cellular apoptosis as a mechanism for radioresistance; however, additional potential death modes involved in modulating radiosensitivity of NPC have not been explored.
Methods: Pyroptosis was assessed by phase-contrast imaging, LDH release assays, live cell imaging, and Western blotting. In vitro and in vivo assays were used to investigate the function of gasdermin E (GSDME) and ovarian tumor family deubiquitinase 4 (OTUD4). NPC tissues were analyzed using Western blotting, immunohistochemistry, and real-time PCR. The molecular mechanism was determined using immunoprecipitation assays and mass spectrometry.
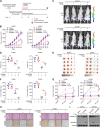
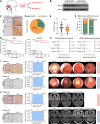
Results: Live cell imaging revealed that 40-75% of irradiation-induced dead NPC cells were pyroptotic cells. Furthermore, irradiation-induced pyroptosis is triggered by GSDME, which are cleaved by activated caspase-3 in the intrinsic mitochondrial pathway. Additionally, GSDME was significantly downregulated in radioresistant NPC specimens. Low GSDME expression was a predictor of worse prognosis and conferred NPC radioresistance both in vitro and in vivo. Mechanistically, OTUD4 deubiquitinated and stabilized GSDME, enhancing radiosensitivity of NPC cells by promoting pyroptosis. Clinically, OTUD4 was significantly correlated with GSDME in NPC biopsies, and patients with low expression of both OTUD4 and GSDME suffered the worst radiotherapy response and survival.
Conclusions: GSDME-dependent pyroptosis is a critical determinant of radiosensitivity in NPC, and is modulated by OTUD4 via deubiquitinating and stabilizing GSDME. These findings reveal a promising novel direction to investigate radioresistance and suggest potential therapeutic targets for sensitizing NPC to radiotherapy.
Keywords: GSDME; Nasopharyngeal carcinoma; OTUD4; Pyroptosis; Radioresistance.
© 2022. The Author(s).
Conflict of interest statement
Authors declare that they have no competing interests.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

