The flutemetamol analogue cyano-flutemetamol detects myocardial AL and ATTR amyloid deposits: a post-mortem histofluorescence analysis
- PMID: 36411500
- PMCID: PMC10199962
- DOI: 10.1080/13506129.2022.2141623
The flutemetamol analogue cyano-flutemetamol detects myocardial AL and ATTR amyloid deposits: a post-mortem histofluorescence analysis
Abstract
Background: [18F]flutemetamol is a PET radioligand used to image brain amyloid, but its detection of myocardial amyloid is not well-characterized. This histological study characterized binding of fluorescently labeled flutemetamol (cyano-flutemetamol) to amyloid deposits in myocardium.
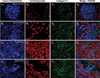
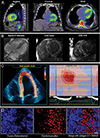
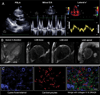
Methods: Myocardial tissue was obtained post-mortem from 29 subjects with cardiac amyloidosis including transthyretin wild-type (ATTRwt), hereditary/variant transthyretin (ATTRv) and immunoglobulin light-chain (AL) types, and from 10 cardiac amyloid-free controls. Most subjects had antemortem electrocardiography, echocardiography, SPECT and cardiac MRI. Cyano-flutemetamol labeling patterns and integrated density values were evaluated relative to fluorescent derivatives of Congo red (X-34) and Pittsburgh compound-B (cyano-PiB).
Results: Cyano-flutemetamol labeling was not detectable in control subjects. In subjects with cardiac amyloidosis, cyano-flutemetamol labeling matched X-34- and cyano-PiB-labeled, and transthyretin- or lambda light chain-immunoreactive, amyloid deposits and was prevented by formic acid pre-treatment of myocardial sections. Cyano-flutemetamol mean fluorescence intensity, when adjusted for X-34 signal, was higher in the ATTRwt than the AL group. Cyano-flutemetamol integrated density correlated strongly with echocardiography measures of ventricular septal thickness and posterior wall thickness, and with heart mass.
Conclusion: The high selectivity of cyano-flutemetamol binding to myocardial amyloid supports the diagnostic utility of [18F]flutemetamol PET imaging in patients with ATTR and AL types of cardiac amyloidosis.
Keywords: amyloid; cardiac; flutemetamol; lambda light chain; positron emission tomography; transthyretin.
Conflict of interest statement
Disclosure statement
MDI served as a consultant and received research funding from GE Healthcare. SD received research funding from GE Healthcare. JD and GF are employees of GE Healthcare. EEA, RFP and VLV have nothing to disclose.
Figures






Similar articles
-
Post-mortem analyses of PiB and flutemetamol in diffuse and cored amyloid-β plaques in Alzheimer's disease.Acta Neuropathol. 2020 Oct;140(4):463-476. doi: 10.1007/s00401-020-02175-1. Epub 2020 Aug 9. Acta Neuropathol. 2020. PMID: 32772265 Free PMC article.
-
Amyloid PET imaging in cardiac amyloidosis: a pilot study using 18F-flutemetamol positron emission tomography.Ann Nucl Med. 2019 Aug;33(8):624-628. doi: 10.1007/s12149-019-01372-7. Epub 2019 May 28. Ann Nucl Med. 2019. PMID: 31140154
-
Quantification of cardiac amyloid with [18F]Flutemetamol in patients with V30M hereditary transthyretin amyloidosis.Amyloid. 2020 Sep;27(3):191-199. doi: 10.1080/13506129.2020.1760237. Epub 2020 May 13. Amyloid. 2020. PMID: 32400202
-
Transthyretin: Its function and amyloid formation.Neurochem Int. 2022 May;155:105313. doi: 10.1016/j.neuint.2022.105313. Epub 2022 Feb 23. Neurochem Int. 2022. PMID: 35218869 Review.
-
Advances in PET-Based Cardiac Amyloid Radiotracers.Curr Cardiol Rep. 2020 May 19;22(6):40. doi: 10.1007/s11886-020-01284-3. Curr Cardiol Rep. 2020. PMID: 32430600 Review.
Cited by
-
Cardiac amyloidosis: Innovations in diagnosis and treatment.Eur Heart J Suppl. 2025 Feb 19;27(Suppl 1):i88-i97. doi: 10.1093/eurheartjsupp/suae111. eCollection 2025 Feb. Eur Heart J Suppl. 2025. PMID: 39980786 Free PMC article.
-
Positron emission tomography in cardiac amyloidosis: current evidence and future directions.Heart Fail Rev. 2025 May;30(3):605-618. doi: 10.1007/s10741-025-10493-3. Epub 2025 Feb 10. Heart Fail Rev. 2025. PMID: 39924609 Free PMC article. Review.
-
Enhanced diagnostic and prognostic assessment of cardiac amyloidosis using combined 11C-PiB PET/CT and 99mTc-DPD scintigraphy.Eur J Nucl Med Mol Imaging. 2025 Jul;52(9):3321-3332. doi: 10.1007/s00259-025-07157-7. Epub 2025 Feb 28. Eur J Nucl Med Mol Imaging. 2025. PMID: 40019577 Free PMC article.
References
-
- Lutter L, Aubrey LD, Xue WF. On the Structural Diversity and Individuality of Polymorphic Amyloid Protein Assemblies. J Mol Biol. 2021;433(20):167124. - PubMed
-
- Sipe JD, Benson MD, Buxbaum JN, et al. Amyloid fibril proteins and amyloidosis: chemical identification and clinical classification International Society of Amyloidosis 2016 Nomenclature Guidelines. Amyloid. 2016;23(4):209–213. - PubMed
-
- Shah KB, Inoue Y, Mehra MR. Amyloidosis and the heart: a comprehensive review. Arch Intern Med. 2006;166(17):1805–1813. - PubMed
-
- Smith TJ, Kyle RA, Lie JT. Clinical significance of histopathologic patterns of cardiac amyloidosis. Mayo Clin Proc. 1984;59(8):547–555. - PubMed
-
- Larsen BT, Mereuta OM, Dasari S, et al. Correlation of histomorphological pattern of cardiac amyloid deposition with amyloid type: a histological and proteomic analysis of 108 cases. Histopathology. 2016;68(5):648–656. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
