Remimazolam: An Updated Review of a New Sedative and Anaesthetic
- PMID: 36411859
- PMCID: PMC9675580
- DOI: 10.2147/DDDT.S384155
Remimazolam: An Updated Review of a New Sedative and Anaesthetic
Abstract
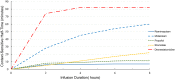
Remimazolam (CNS7056) is a novel benzodiazepine for intravenous sedation; it has an ultra-short duration of action and was recently approved for use in procedural sedation and general anaesthesia. It acts on γ-aminobutyric acid type A receptors and is rapidly converted into an inactive metabolite by tissue esterase enzymes. Remimazolam has been successfully used in endoscopic inspection or surgery and general anaesthesia induction and maintenance with fast and predictable onset and recovery times, high procedure success rates, and minor respiratory and hemodynamic fluctuations and without serious drug-related adverse reactions. If needed, the effects of remimazolam can be reversed by flumazenil, which allows prompt termination of sedation. Although remimazolam has great potential for sedation in patients admitted to intensive care units, future studies are needed to evaluate its efficacy and safety in patients requiring sedation for a long period, and numerous studies are warranted to explore the optimal dose in different application scenarios. The review aimed to provide an introduction to the process of remimazolam synthesis and its current clinical uses and future clinical developments.
Keywords: ICU; endoscopy; general anaesthesia; sedation.
© 2022 Hu et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest in this work.
Figures

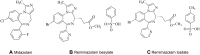

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

