OAE-based data mining and modeling analysis of adverse events associated with three licensed HPV vaccines
- PMID: 36411908
- PMCID: PMC9674903
- DOI: 10.1016/j.heliyon.2022.e11515
OAE-based data mining and modeling analysis of adverse events associated with three licensed HPV vaccines
Abstract
Purpose: Three licensed human papillomavirus (HPV) vaccines (Cervarix, Gardasil, and Gardasil 9) have been effectively used to prevent infection with oncogenic HPV types; however, many adverse events (AEs) have also been reported following their vaccinations. We assessed AE profiles after receiving the HPV vaccines based on the reported data from Vaccine Adverse Event Reporting System (VAERS).
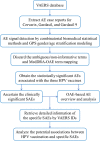
Methods: The AE data associated with Cervarix, Gardasil, and Gardasil 9 were retrieved from VAERS database respectively. The combinatorial biomedical statistical methods were used to identify the statistically significant AEs. The Gamma-Poisson Shrinker (GPS) model with gender/age stratification was applied to ascertain the serious adverse events (SAEs) related to the three licensed HPV vaccines. The AE profiles were classified and represented by the Ontology of Adverse Events (OAE) for further analysis.
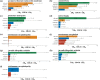
Results: As of July 31, 2020, VAERS recorded 3,112, 31,606, and 6,872 AE case reports for Cervarix, Gardasil, and Gardasil 9, respectively. Our Frequentist statistical methods identified 135 Cervarix-enriched AEs, 55 Gardasil-enriched AEs, and 17 Gardasil 9-enriched AEs. Based on the OAE hierarchical classification, these AEs were clustered in the AEs related to behavioral and neurological conditions, immune system, nervous system, and reproductive system. Combined with GPS modeling, 46 unique statistically significant SAEs were founded to be associated with at least one of the three vaccines.
Conclusions: Our study led to the better understanding of the AEs associated with the licensed HPV vaccines. The hypotheses on the cause and effect relationships between the HPV vaccination and specific AEs deserve further epidemiological investigations as well as clinical trial studies.
Keywords: Adverse event; Gamma-Poisson shrinker; Human papillomavirus; Ontology of adverse events; Vaccine; Vaccine adverse event reporting system (VAERS).
© 2022 The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Profiling COVID-19 Vaccine Adverse Events by Statistical and Ontological Analysis of VAERS Case Reports.Front Pharmacol. 2022 Jun 24;13:870599. doi: 10.3389/fphar.2022.870599. eCollection 2022. Front Pharmacol. 2022. PMID: 35814246 Free PMC article.
-
Ontology-based combinatorial comparative analysis of adverse events associated with killed and live influenza vaccines.PLoS One. 2012;7(11):e49941. doi: 10.1371/journal.pone.0049941. Epub 2012 Nov 28. PLoS One. 2012. PMID: 23209624 Free PMC article.
-
Statistical and Ontological Analysis of Adverse Events Associated with Monovalent and Combination Vaccines against Hepatitis A and B Diseases.Sci Rep. 2016 Oct 3;6:34318. doi: 10.1038/srep34318. Sci Rep. 2016. PMID: 27694888 Free PMC article.
-
Ontology-Based Vaccine Adverse Event Representation and Analysis.Adv Exp Med Biol. 2017;1028:89-103. doi: 10.1007/978-981-10-6041-0_6. Adv Exp Med Biol. 2017. PMID: 29058218 Review.
-
Human papillomavirus vaccines.Ann Pharmacother. 2006 Jul-Aug;40(7-8):1344-52. doi: 10.1345/aph.1G723. Epub 2006 Jul 18. Ann Pharmacother. 2006. PMID: 16849621 Review.
Cited by
-
Investigating the Efficacy of HPV Vaccines in Preventing Cervical Cancer from 2006 to 2018 in the US: A SEER Data Set Analysis.Rev Recent Clin Trials. 2023;18(3):214-222. doi: 10.2174/1574887118666230410093715. Rev Recent Clin Trials. 2023. PMID: 37046192 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

