Multiple sclerosis clinical decision support system based on projection to reference datasets
- PMID: 36412095
- PMCID: PMC9735373
- DOI: 10.1002/acn3.51649
Multiple sclerosis clinical decision support system based on projection to reference datasets
Abstract
Objective: Multiple sclerosis (MS) is a multifactorial disease with increasingly complicated management. Our objective is to use on-demand computational power to address the challenges of dynamically managing MS.
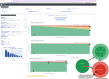
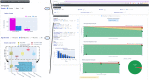
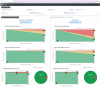
Methods: A phase 3 clinical trial data (NCT00906399) were used to contextualize the medication efficacy of peg-interferon beta-1a vs placebo on patients with relapsing-remitting MS (RRMS). Using a set of reference patients (PORs), selected based on adequate features similar to those of an individual patient, we visualize disease activity by measuring the percentage of relapses, accumulation of new T2 lesions on MRI, and worsening EDSS during the clinical trial.
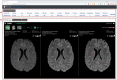
Results: We developed MS Vista, a functional prototype of clinical decision support system (CDSS), with a user-centered design and distributed infrastructure. MS Vista shows the medication efficacy of peginterferon beta-1a versus placebo for each individual patient with RRMS. In addition, MS Vista initiated the integration of a longitudinal magnetic resonance imaging (MRI) viewer and interactive dual physician-patient data display to facilitate communication.
Interpretation: The pioneer use of PORs for each individual patient enables personalized analytics sustaining the dialog between neurologists, patients and caregivers with quantified evidence.
© 2022 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Pierre‐Antoine Gourraud is the founder of MethodOmics (2008) (
David‐Axel Laplaud receives consulting fees from Alexion, Biogen, BMS, Merck, Novartis, Sanofi and Roche.
Figures


References
-
- Eshaghi A, Young A, Wijertane P, et al. Defining multiple sclerosis subtypes using machine learning. medRxiv. 2020;19011080. doi:10.1101/19011080 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
