Coordination of Cilia Movements in Multi-Ciliated Cells
- PMID: 36412641
- PMCID: PMC9680496
- DOI: 10.3390/jdb10040047
Coordination of Cilia Movements in Multi-Ciliated Cells
Abstract
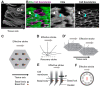
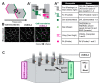
Multiple motile cilia are formed at the apical surface of multi-ciliated cells in the epithelium of the oviduct or the fallopian tube, the trachea, and the ventricle of the brain. Those cilia beat unidirectionally along the tissue axis, and this provides a driving force for directed movements of ovulated oocytes, mucus, and cerebrospinal fluid in each of these organs. Furthermore, cilia movements show temporal coordination between neighboring cilia. To establish such coordination of cilia movements, cilia need to sense and respond to various cues, including the organ's orientation and movements of neighboring cilia. In this review, we discuss the mechanisms by which cilia movements of multi-ciliated cells are coordinated, focusing on planar cell polarity and the cytoskeleton, and highlight open questions for future research.
Keywords: cytoskeleton; motile cilia; multi-ciliated cells; planar cell polarity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

