Polyzwitterionic Coating of Porous Adsorbents for Therapeutic Apheresis
- PMID: 36412857
- PMCID: PMC9680258
- DOI: 10.3390/jfb13040216
Polyzwitterionic Coating of Porous Adsorbents for Therapeutic Apheresis
Abstract
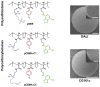
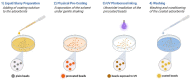
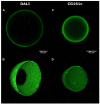
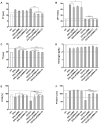
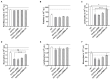
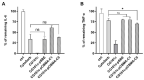
Adsorbents for whole blood apheresis need to be highly blood compatible to minimize the activation of blood cells on the biomaterial surface. Here, we developed blood-compatible matrices by surface modification with polyzwitterionic polysulfobetainic and polycarboxybetainic coatings. Photoreactive zwitterionic terpolymers were synthesized by free-radical polymerization of zwitterionic, photoreactive, and fluorescent monomers. Upon UV irradiation, the terpolymers were photodeposited and mutually crosslinked on the surface of hydrophobic polystyrene-co-divinylbenzene and hydrophilic polyacrylamide-co-polyacrylate (DALI) beads. Fluorescent microscopy revealed coatings with an average thickness of 5 µm, which were limited to the bead surface. Blood compatibility was assessed based on polymer-induced hemolysis, coagulation parameters, and in vitro tests. The maintenance of the adsorption capacity after coating was studied in human whole blood with cytokines for polystyrene beads (remained capacity 25-67%) and with low-density lipoprotein (remained capacity 80%) for polyacrylate beads. Coating enhanced the blood compatibility of hydrophobic, but not of hydrophilic adsorbents. The most prominent effect was observed on coagulation parameters (e.g., PT, aPTT, TT, and protein C) and neutrophil count. Polycarboxybetaine with a charge spacer of five carbons was the most promising polyzwitterion for the coating of adsorbents for whole blood apheresis.
Keywords: adsorbents; blood compatibility; coat; extracorporeal therapies; polyzwitterions.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
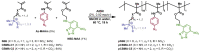

Similar articles
-
The impact of citrate concentration on adhesion of platelets and leukocytes to adsorbents in whole blood lipoprotein apheresis.J Clin Apher. 2017 Dec;32(6):375-383. doi: 10.1002/jca.21519. Epub 2016 Nov 17. J Clin Apher. 2017. PMID: 27859540
-
DALI-the first human whole-blood low-density lipoprotein and lipoprotein (a) apheresis system in clinical use: procedure and clinical results.Eur J Clin Invest. 1998 Dec;28(12):994-1002. doi: 10.1046/j.1365-2362.1998.00395.x. Eur J Clin Invest. 1998. PMID: 9893010 Clinical Trial.
-
Heparin as a molecular spacer immobilized on microspheres to improve blood compatibility in hemoperfusion.Carbohydr Polym. 2019 Feb 1;205:89-97. doi: 10.1016/j.carbpol.2018.08.067. Epub 2018 Sep 1. Carbohydr Polym. 2019. PMID: 30446153
-
Defining the role of lipoprotein apheresis in the management of familial hypercholesterolemia.Am J Cardiovasc Drugs. 2011 Dec 1;11(6):363-70. doi: 10.2165/11594970-000000000-00000. Am J Cardiovasc Drugs. 2011. PMID: 22149315 Review.
-
Recent advances in therapeutic apheresis.J Artif Organs. 2003;6(1):1-8. doi: 10.1007/s100470300000. J Artif Organs. 2003. PMID: 14598117 Review.
Cited by
-
Aminolysis-Based Zwitterionic Immobilization on Polyethersulfone Membranes for Enhanced Hemocompatibility: Experimental, Computational, and Ex Vivo Investigations.Biomimetics (Basel). 2024 May 27;9(6):320. doi: 10.3390/biomimetics9060320. Biomimetics (Basel). 2024. PMID: 38921200 Free PMC article.
-
Polycarboxybetaine in advanced drug delivery systems: From structure-function relationship to therapeutic applications.Int J Pharm X. 2025 Mar 29;9:100329. doi: 10.1016/j.ijpx.2025.100329. eCollection 2025 Jun. Int J Pharm X. 2025. PMID: 40236609 Free PMC article. Review.
References
-
- Mickiewicz A., Borowiec-Wolna J., Bachorski W., Gilis-Malinowska N., Gałąska R., Raczak G., Chmara M., Wasąg B., Jaguszewski M.J., Fijałkowski M., et al. Long-Term Lipoprotein Apheresis in the Treatment of Severe Familial Hypercholesterolemia Refractory to High Intensity Statin Therapy: Three Year Experience at a Lipoprotein Apheresis Center. Cardiol. J. 2019;26:669–679. doi: 10.5603/CJ.a2018.0100. - DOI - PMC - PubMed
-
- Ankawi G., Neri M., Zhang J., Breglia A., Ricci Z., Ronco C. Extracorporeal Techniques for the Treatment of Critically Ill Patients with Sepsis beyond Conventional Blood Purification Therapy: The Promises and the Pitfalls 11 Medical and Health Sciences 1107 Immunology. Crit. Care. 2018;22:262. doi: 10.1186/s13054-018-2181-z. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

